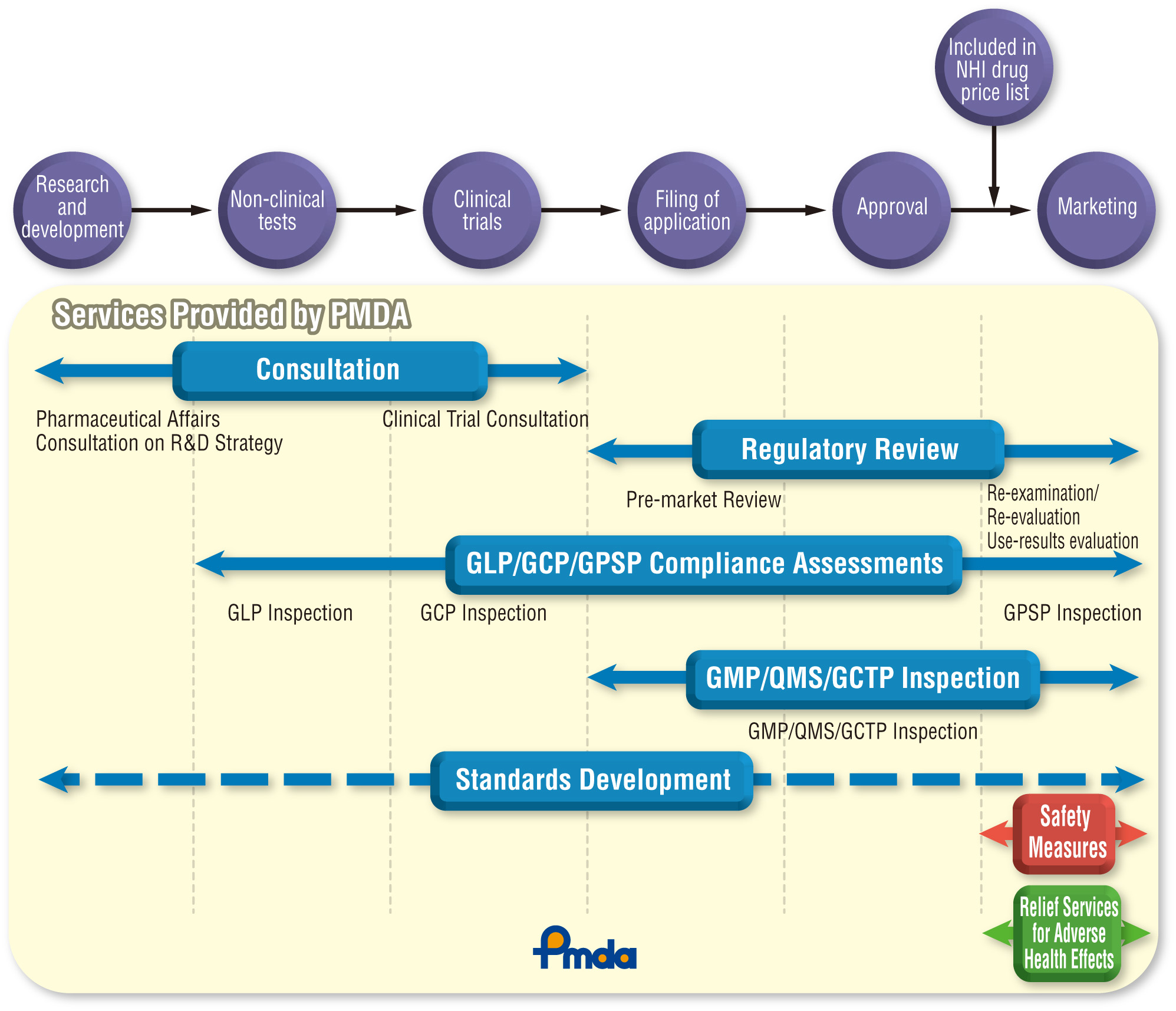
During the review process, PMDA evaluates the quality,efficacy, and safety of drugs, medical devices, and cellular and tissue-based products in light of current scientific and technological standards. In addition, PMDA’s reviews and related services consist of various activities, such as “consultations” providing advice in relation to regulatory submission, GLP/GCP/GPSP inspections to ensure the submitted data are in compliance with the ethical and scientific standards, and GMP/QMS/GCTP inspections to ensure quality management of the manufacturing facility for the product submitted for approval.
Back
Back
-
Regulations & Services of PMDA
Information about Approved Products in Japan
International Activities
Safety Information
Advanced Efforts
-
Information for approved products in Japan
Safety information
-
Development of Drugs, Medical devices, Regenerative medicines and in Vitro diagnostics
Information for approved products in Japan
Regulatory science
-
Regulations and services of PMDA
Information for approved products in Japan
Procedures in Japan
Advanced efforts
Back
-
Regulations and Services of PMDA
Information for apporoved products in Japan
Safety information
Procedures in Japan
-
Regulations and Services of PMDA
Information for apporoved products in Japan
Safety information
Procedures in Japan
-
Reviews and Related Services
Regulatory Procedures
Post-marketing Safety Measures
-
Reviews and Related Services
Post-marketing Safety
Measures
- Home
- Reviews and Related Services
- Outline
Reviews and Related Services