The PMDA has started efforts to develop a framework for collecting and evaluating data on an operating status of implantable medical devices based on the first mid-term plan. Therefore the PMDA launched a project of the Japanese registry for Mechanically Assisted Circulatory Support (J-MACS)as part of new efforts for post-marketing safety measures for medical devices. The project is ongoing under the second mid-term plan.
What is J-MACS ?
- J-MACS is a post-market data-collection project related to ventricular assist device (VAD) in Japan.
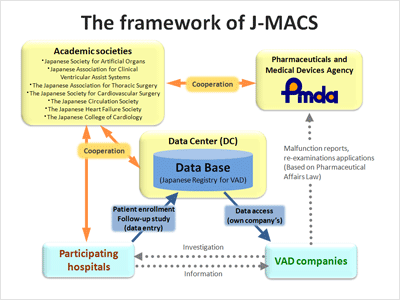
- The implementation plan was developed as an observational study in collaboration with 7 academic societies, participating hospitals (6 at start), the relevant VAD companies and PMDA under the cooperation among industry-government-academia.
- Membership in J-MACS is one of the essential conditions for authorization of hospitals to use implantable VADs under the national medical insurance coverage.
- All hospitals which use implantable VADs provide information about the post-marketing data of the VADs approved in Japan, which is on a long term basis through WEB based data entry system.
- The VAD companies utilize the patients' data collected through J-MACS to report medical device malfunction and conduct post-marketing surveillance required under the Pharmaceutical affairs law.
The framework of J-MACS
Objective
- J-MACS will be useful for improving clinical assessment/management, treatment, and technologies for severe heart failure patients.
- The data will be beneficial for assuring patient safety in developing next-generation devices by clarifying risks and benefits.
- Data assessment will promote implementation of appropriate safety measures.
Prospective Design
- J-MACS is a prospective registry in a post-marketing observational research that collect clinical data, including follow up, essentially as it happens.
Eligibility : Inclusion criteria
- Patients who receive a durable VAD which is approved in Japan.
- Patients who receive a VAD after hospital activated.
- Patients who have signed informed consent for the registry.
Outline of the organization
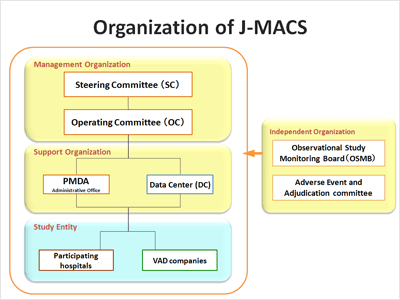
J-MACS has established the Steering Committee consisting of representatives from academic societies and a manufacturers association and the Operating Committee consisting of a principal investigator (PI), co-PIs, experts from hospitals and the VAD companies.
In addition, as independent organizations to oversee the registry, Observational Study Monitoring Board and Adverse Event and Adjudication committee have been established.
Organization of J-MACS
The number of Patient Enrollment
Statistical Report
Presentations
- Initial Report of J-MACS
: The poster presented by the principal investigator at the ISHLT 2012 Scientific Sessions - J-MACS
: The brochure of the J-MACS presented at the JSAO / IFAO Joint International Congress 2013 - J-MACS Registry Update
: The document presented by the principal investigator at the IMACS Registry Meeting at the ISHLT 2015 Scientific Sessions
Reference
Mid-term Plan of the Pharmaceuticals and Medical Devices Agency (excerption)
Construct a system for gathering and evaluating data on the operational status of high risk, implantable tracking medical devices (implantable ventricular-assist devices), such as the occurrence rate of malfunctions over time, and appropriately utilize such system in the development of safety measures.