- Introduction
- Eligible Participants
- Date / Time
- Program Agenda
- Registration
- Fee
- Location
- Access
- Contact Us
Introduction
The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to announce its fifth training seminar for international regulatory authorities scheduled for October 6-10, 2014. The seminar is established for the exchange of drug and medical device regulatory information between the PMDA and its counterpart agencies all over the world. The seminar is not open to the pharmaceutical industry or non-regulatory authorities. The seminar is not intended for Japan government employees.
The 5th PMDA Training Seminar is designed for officials who are engaged in review of new drugs (including biopharmaceuticals and tissue and cellular products). In this program, PMDA experts will present
1) PMDA's role in regulation
2) Japanese pharmaceutical regulations
3) Review of new drugs and their case studies
The training will be conducted in English. All participants are expected to actively participate in all of the sessions.
Eligible Participants
Participation in the PMDA 5th Training Seminar is limited to officials who are engaged in review of new drugs (including biopharmaceuticals and tissue and cellular products). Confirmation of registration and additional meeting information will be sent to the approved participants.
Date / Time
October 6, 2014, 10:00 a.m. to October 10, 2014 15:00 p.m.
Program Agenda (Draft)
Day 1 (Monday, 10/6, 2014)
| Time | Topic |
| 10:00 - 10:10 | Welcome and Introduction |
| 10:10 - 10:20 | Training Overview / Schedule |
| 10:20 - 11:00 | Overview of PMDA |
| 11:00 - 12:00 | Conversation Hour |
| 12:00 - 13:00 | Lunch on Your own |
| 13:00 - 14:00 | Pharmaceutical Affairs Act |
| 14:00 - 15:00 | Review of Pharmaceuticals |
| 15:00 - 15:30 | Break |
| 15:30 - 16:15 | GCP |
| 16:15 - 17:00 | GMP |
| 17:00 - 18:00 | Japanese Pharmacopoeia / Master File System |
Day 2 (Tuesday, 10/7, 2014)
| 10:00 - 10:45 | Quality Standards (chemicals) |
| 10:45 - 11:30 | Quality Standards (biopharmaceuticals) |
| 11:30 - 12:15 | Toxicology |
| 12:15 - 13:15 | Lunch on Your own |
| 13:15 - 14:00 | Pharmacology |
| 14:00 - 14:45 | Pharmacokinetics |
| 14:45 - 15:15 | Break |
| 15:15 - 16:00 | Clinical Data |
| 16:00 - 16:45 | Cardiovascular Risk |
| 16:45 - 17:30 | Statistics |
Day 3 (Wednesday, 10/8, 2014)
| 10:00 - 10:45 | Clinical Trials and Consultation |
| 10:45 - 11:45 | Cross-sectional Projects |
| 11:45 - 13:15 | Lunch on Your own 12:20-12:50 lunch time seminar by Ms. Li Fung Peck (Singapore HSA) |
| 13:15 - 17:00 | Group Work 1 (Pharmacokinetics in Drug Development) |
| 17:00 - 18:00 | Wrap up: Group Work 1 |
Day 4 (Thursday, 10/9, 2014)
| 10:00 - 10:45 | Tissue and Cellular products |
| 10:45 - 11:00 | Break |
| 11:00 - 11:45 | Biosimilar |
| 11:45 - 13:15 | Lunch on Your own 12:20-12:50 lunch time seminar by Ms. Shanti Marlina Sibuea (Indonesia NADFC) |
| 13:15 - 17:00 | Group Work 2 (Product Review Regarding Type 2 Diabetes Mellitus) PMDA will explain key points in product reviews using review reports and relevant guidelines as references. |
| 17:00 - 18:00 | Wrap up: Group Work 2 |
Day 5 (Friday, 10/10, 2014)
| 10:00 - 11:00 | Post-marketing Safety Measures for Pharmaceuticals |
| 11:00 - 11:15 | Break |
| 11:15 - 12:00 | Relief Services |
| 12:00 - 13:00 | Lunch on Your own |
| 13:00 - 15:00 | Wrap up of the Training Seminar |
Note: The program is tentative and will be updated.
Registration
Registration, request should include the following information about the proposed attendee:
Application form is in Word / PDF format. Please download here. "Application form", "Application form"
Please fill in all the necessary items on the application form and send it by e-mail at 5th_training_seminar@pmda.go.jp
Registration will close September 5, 2014. Please be sure to allow enough time to obtain your visa.
Fee
There is no registration fee for this seminar.
However, attendees are responsible for their own travel, lodging and food expenses.
Location
Pharmaceuticals and Medical Devices Agency (PMDA):
Shin-kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013, Japan
Access
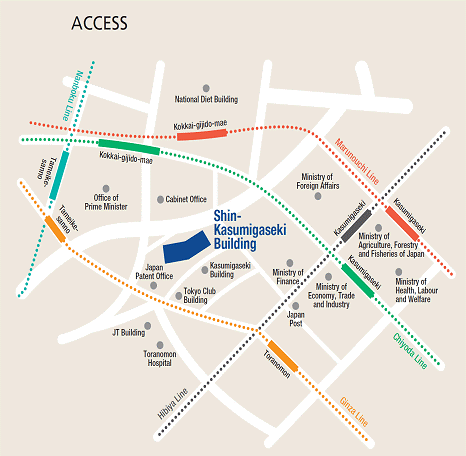
Please use public transportation. Nearest Tokyo Metro Subway Stations:
- 5 minutes walk from Exit 11 of Toranomon Station on the Ginza Line
- 8 minutes walk from Exit 3 of Kokkai-gijido-mae Station, on the Marunouchi Line, Chiyoda Line
- 8 minutes walk from Exit A13 of Kasumigaseki Station, on the Marunouchi Line, Chiyoda Line, Hibiya Line
- 10 minutes walk from Exit 8 of Tameike-sanno Station, on the Ginza Line, Nanboku Line
Contact Us
For more information, please contact:
Tamami Fukushi, Ph.D.
Division of Planning and Coordination
Office of International Programs
Pharmaceuticals and Medical Devices Agency
E-mail: 5th_training_seminar@pmda.go.jp
