Introduction
The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to announce the holding of the “PMDA-ATC Pharmaceuticals Review Seminar 2016" for officials from overseas regulatory authorities on July 25 - 29, 2016.
The Seminar will be offered by the Asia Training Center for Pharmaceuticals and Medical Devices Regulatory Affairs (PMDA-ATC) at PMDA in Tokyo.
At the Seminar, the details of regulatory works for pharmaceuticals including consultation and product reviews will be shared. Case studies and discussions among participants are planned as well.
The aims of the Seminar are to provide chances for the participants to refer back to their work and to find any additional opportunity for enhancement of the participant's regulatory system.
The seminar is not open to the industry or non-regulatory authorities. The seminar is not intended for Japanese government employees, either.
Key Seminar Objective
- To learn basics of regulations and regulatory organization
- To learn the key regulatory flow of the product development to post-approval
The Seminar will focus on the following key regulatory activities.
- Consultations (Scientific Advice)
- Product review
- To make use of the learnings from case studies and discussions and reflect it
Who should apply
- Officials from regulatory authorities who are currently engaged in review of pharmaceuticals.
A basic knowledge of the regulations pertaining to the pharmaceutical product review
in his/her organization is required.
The training will be provided in English (with simultaneous translation in some sessions).
All participants are expected to actively participate in all of the sessions.
Date / Time
July 25 to 29, 2016. See attached program for draft schedule.
Program (subject to change)
See attached for the draft program
Registration
- Registration request should be made by submitting the completed application form.
- Please fill in all the necessary items on the application form and send it by e-mail at PMDA-ATC@pmda.go.jp
- Registration will close on June 24, 2016.
- Early registration is recommended. The registration may close before the deadline depending on the number of application. Also, please be sure to allow enough time to obtain the visa.
- If the number of applications exceeds the capacity, the number of participants from each country may be limited.
- Confirmation of the registration and additional meeting information will be sent to the approved participants after the close of registration.
Fee
There is no registration fee for this seminar.
Attendees are responsible for their own travel, accommodation and food expenses
Location
Pharmaceuticals and Medical Devices Agency (PMDA):
Shin-kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku,Tokyo 100-0013, Japan
Access
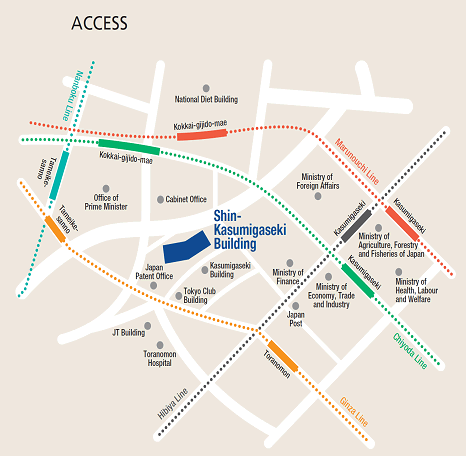
Please use public transportation. Nearest Tokyo Metro Subway Stations:
- 5 minutes’ walk from Exit 11 of Toranomon Station on the Ginza Line
- 8 minutes’ walk from Exit 3 of Kokkai-gijido-mae Station, on the Marunouchi Line, Chiyoda Line
- 8 minutes’ walk from Exit A13 of Kasumigaseki Station, on the Marunouchi Line, Chiyoda Line, Hibiya Line
- 10 minutes’ walk from Exit 8 of Tameike-sanno Station, on the Ginza Line, Nanboku Line
Contact Us
For more information, please contact:
Secretariat, PMDA Asia Training Center
E-mail: PMDA-ATC@pmda.go.jp
Office of International Cooperation
Pharmaceuticals and Medical Devices Agency
Shin-Kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan
