Introduction
The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to announce the holding of the "PMDA-ATC Multi-Regional Clinical Trial (MRCT) Seminar 2018" for new drug application reviewers from overseas regulatory authorities. The Seminar will be offered by the Asia Training Center for Pharmaceuticals and Medical Devices Regulatory Affairs (PMDA-ATC) at PMDA Office in Tokyo, from January 15-18, 2018.
This Seminar is held as a workshop of the Asia-Pacific Economic Cooperation, Life Sciences Innovation Forum, Regulatory Harmonization Steering Committee (APEC-LSIF-RHSC) Centers of Excellence; however, the Seminar is open to non-APEC economies as well.
With the increase in global product development and the ICH E17 guideline (General principle on planning/designing MRCTs) close to being harmonized, product reviews based on MRCTs driven data are also increasing. Under this situation, it is essential that regulators adequately evaluate the benefit-risk balance while keeping in mind the ethnic factors.
The Seminar aims to promote drug development and convergence of regulations by sharing accumulated experiences of Japan, such as protocol designing and planning of MRCT, clinical data evaluation, clinical operation, GCP inspections, post-marketing issues and safety measures for approved products based on MRCT. The Seminar will be led not only by PMDA reviewers but also by representatives from academia and industry, allowing participants to think and discuss from multiple perspectives. As changes from the previous program, PMDA adds new sessions which are clinical site tour and introduction of review systems and regulations by participants in the program for this fiscal year.
The Seminar is not open to the industry or non-regulatory authorities. The Seminar is not intended for Japanese government employees, either.
Participation from many regulatory authorities is welcomed.

Key Seminar Objectives
- To learn current situation surrounding MRCTs
- To learn the key regulatory flow of the drug development to post-approval utilizing MRCTs
- To obtain an opportunity to discuss how to make regulatory decisions based on specific cases
- To build skilled human capacity in regulatory science to promote and facilitate MRCTs
- To enhance regulatory cooperation in the APEC region on the evaluation and regulation of MRCTs
- To increase the acceptability of MRCT data by multiple regulatory authorities
Who should apply
- Senior clinical reviewers with at least 3 years of hands-on experience in the assessment of clinical trial applications, benefit-risk profiles for market approvals and post-market activities
- The training will be provided in English (with consecutive translation in some sessions).
- All participants are expected to actively participate in all of the sessions.
Date / Time
January 15 to 18, 2018.
Program Outline (subject to change)
See attached for the draft program
Registration (Registration is closed.)
- Registration request should be made by filling in all the necessary items on the application form and sending it by e-mail to the e-mail address shown in “Contact Us” below.
- Registration will close on October 13, 2017.
- Early registration is recommended. The registration may close before the deadline depending on the number of application. Also, please be sure to allow enough time to obtain the visa for entry to Japan.
- If the number of applications exceeds the capacity, the number of participants from each country may be limited.
- Confirmation of the registration and additional information will be sent to the approved participants after the close of registration.
Fee
There is no registration fee for this Seminar.
Information on travel and hotel reservation assistance will be announced to the approved participants.
Location
Pharmaceuticals and Medical Devices Agency (PMDA):
Shin-kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku,Tokyo 100-0013, Japan
Access
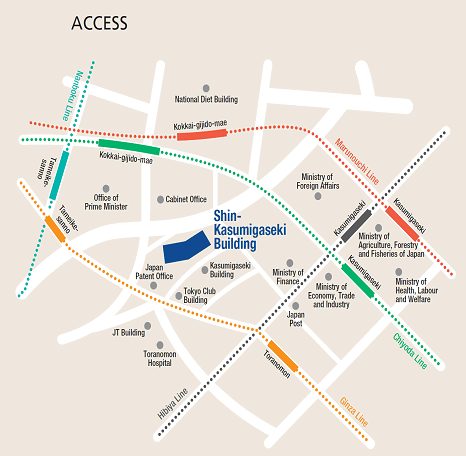
Please use public transportation. Nearest Tokyo Metro Subway Stations:
- 5 minutes’ walk from Exit 11 of Toranomon Station on the Ginza Line
- 8 minutes’ walk from Exit 3 of Kokkai-gijido-mae Station, on the Marunouchi Line, Chiyoda Line
- 8 minutes’ walk from Exit A13 of Kasumigaseki Station, on the Marunouchi Line, Chiyoda Line, Hibiya Line
- 10 minutes’ walk from Exit 8 of Tameike-sanno Station, on the Ginza Line, Nanboku Line
Contact Us
For more information, please contact:
Secretariat, PMDA Asia Training Center
E-mail: PMDA-ATC●pmda.go.jp
Office of International Cooperation
Pharmaceuticals and Medical Devices Agency
Shin-Kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan
(Note: For the purpose of security, @ in the e-mail addresses are replaced with ●. Please replace ● with @ when you send an e-mail.)
