Introduction
The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to announce the holding of the "PMDA-ATC Medical Devices Seminar 2019" for officials from overseas regulatory authorities who are engaged in the review of medical devices and in vitro diagnostics.
The seminar will be offered by the Asia Training Center for Pharmaceuticals and Medical Devices Regulatory Affairs (PMDA-ATC) at PMDA Office in Tokyo, from November 25 to 29, 2019.
This year’s seminar is held as APEC-LSIF-RHSC's APEC Center of Excellence Pilot Workshop; however, the Seminar is open to non-APEC economies as well*.
The seminar is not open to the industry or non-regulatory authorities. Participation from many regulatory authorities is welcomed.
* APEC-LSIF-RHSC: Asia-Pacific Economic Cooperation (APEC), Life Sciences Innovation Forum (LSIF), Regulatory Harmonization Steering Committee (RHSC)
The seminar will cover wide range of topics, such as regulations, consultations (scientific advices), product reviews, GCP/GLP/QMS, safety measures, standards, etc. Small group discussions among participants, and site visit to manufacturing facility are planned as well.
The aims of the seminar are to provide chances for the participants to refer back to their work and to find any additional opportunity for enhancement of the participant's regulatory system.
Key Seminar Objective
- To learn basics of medical device regulations and the key regulatory flow of the review of medical devices, especially on the following points.
- Product classifications based on risk level
- Scientific reviews
- GCP/GLP/QMS/standards
- Safety measures
- To obtain up-to-date information about international regulatory harmonization effort for medical devices (utilization of international standards, IMDRF, etc.)
- To utilize the knowledge learned from the seminar to enhance the regulatory system in the participant’s own organization.
Who should apply
- Officials from regulatory authorities who are currently engaged in the review of medical devices and in vitro diagnostics.
- Basic knowledge of the regulations pertaining to the medical devices product review in his/her organization is required.
- The training will be provided in English (with consecutive translation in some sessions).
- All participants are expected to actively participate in all of the sessions.
- A representative of each country/region will be required to present the review system for medical devices in his/her own country/region to other participants.
Date
November 25 to 29, 2019
Program (subject to change)
See the draft program (attached)
Registration
Number of participants: approx. 30
Close of registration: (Registration is closed)
- Registration request should be made by filling in all the necessary items including signature by Head of Organization on the application form (attached).Please send the form in both the Word file (without signature) and the PDF file (with signature) by e-mail to the e-mail address shown in “Contact Us” below.
- Early registration is recommended. After the close of registration, we are not able to accept change or addition of participant(s) in principle. Also, please be sure to allow enough time to obtain the visa for entry to Japan.
- If the number of applications exceeds the capacity, the number of participants from each country may be limited. Selection of the participants will take place at the discretion of PMDA based on the information provided in the application form.
- Confirmation of the registration and additional information will be sent to the approved participants after the close of registration.
Fee
There is no registration fee for this seminar.
Information on travel and hotel reservation assistance will be announced to the approved participants.
Location
Pharmaceuticals and Medical Devices Agency (PMDA):
Shin-kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013, Japan
Access
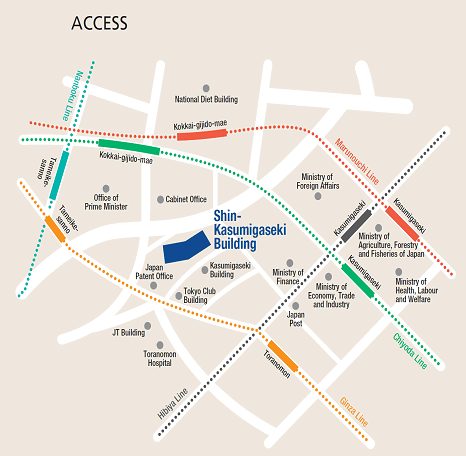
Please use public transportation. Nearest Tokyo Metro Subway Stations:
- 5 minutes' walk from Exit 11 of Toranomon Station on the Ginza Line
- 8 minutes' walk from Exit 3 of Kokkai-gijido-mae Station, on the Marunouchi Line, Chiyoda Line
- 8 minutes' walk from Exit A13 of Kasumigaseki Station, on the Marunouchi Line, Chiyoda Line, Hibiya Line
- 10 minutes' walk from Exit 8 of Tameike-sanno Station, on the Ginza Line, Namboku Line
Contact Us
For more information, please contact:
Secretariat, PMDA Asia Training Center
E-mail: PMDA-ATC●pmda.go.jp
Office of International Cooperation
Pharmaceuticals and Medical Devices Agency
Shin-Kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013, Japan
(Note: For the purpose of security, @ in the e-mail addresses are replaced with ●. Please replace ● with @ when you send an e-mail.)
