Overview
The Japanese medical device Quality Management System requirements are stipulated in MHLW Ministerial Ordinance No. 169 (2004) titled "the Ministerial Ordinance on Standards for Manufacturing Control and Quality Control for Medical Devices and In-Vitro Diagnostics" (hereinafter refered to as MHLW MO169). MHLW MO169 was initially established in 2004 in order to make the medical device QMS requirements harmonized with international standard, ISO13485:2003. The ordinace has been revised several times since its establishment (see the following).
Revision history, MHLW MO169 (only main revision)
| 2004: Initial version published. |
| 2014: The second chapter of the ordinance was revised to more align with ISO13485:2003. The additional requirements to ISO13485:2003 were moved to the third chapter of the ordinance. |
| 2017: Requirements for re-manufacturing single-use device (R-SUD) were incorporated in the ordinace. |
| 2021: The second chapter of the ordinance was revised to align with ISO13485:2016. |
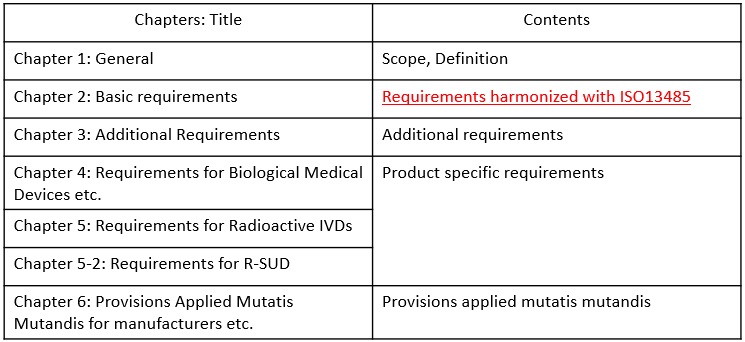
Contents of MHLW MO169
Recent revision
MHLW MO169 was revised to align with ISO13485:2016 in March 26, 2021. The transition period is 3 years. Hence, Marketing Authoriation Holders etc. and Registered Manufacturing Sites must comply with the revised ordinance by March 25, 2024.
Materials
Main requirements of MHLW MO169 are stipulated in the second chapter of the ordinance. The requirements are aligned with ISO13485. The followings are comparison table between ISO13485 and MHLW MO169 Chapter 2.
- MHLW MO169, as revised in 2021 (Current version)
Comparison table between ISO13485:2016 and MHLW MO169 Chapter 2, as revised in 2021 - MHLW MO169, as revised in 2014 (Previous version)
Comparison table between ISO13485:2003 and MHLW MO169 Chapter 2, as revised in 2014
The addtional requirements of MHLW MO169 are stipulated in the third chapter of the ordinance. The followings are translation of the requirements.
- MHLW MO169, as revised in 2021 (Current version)
Tentative translation of MHLW MO169 Chapter 3, as revised in 2021
- MHLW MO169, as revised in 2014 (Previous version)
Tentative translation of MHLW MO169 Chapter 3, as revised in 2014
The product specific requirements of MHLW MO169 are stipulated in the chapter 4, 5,and 5-2 of the ordinance. The followings are translation of the requirements.
- Tentative translation of MHLW MO169 Chapter 4, as revised in 2021
- Tentative translation of MHLW MO169 Chapter 5, as revised in 2021
- Tentative translation of MHLW MO169 Chapter 5-2, as revised in 2021
The followings are translation of the requirements for R-SUDs other than MHLW MO169. Manufacturers shall comply with these requirements as well as MHLW MO169 Chapter 5-2, when they manufacture R-SUDs.
- Tentative translation of MHLW MO1 (Regulations for Enforcement of the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices)
- Standards for Re-manufactured Single-use Medical Devices