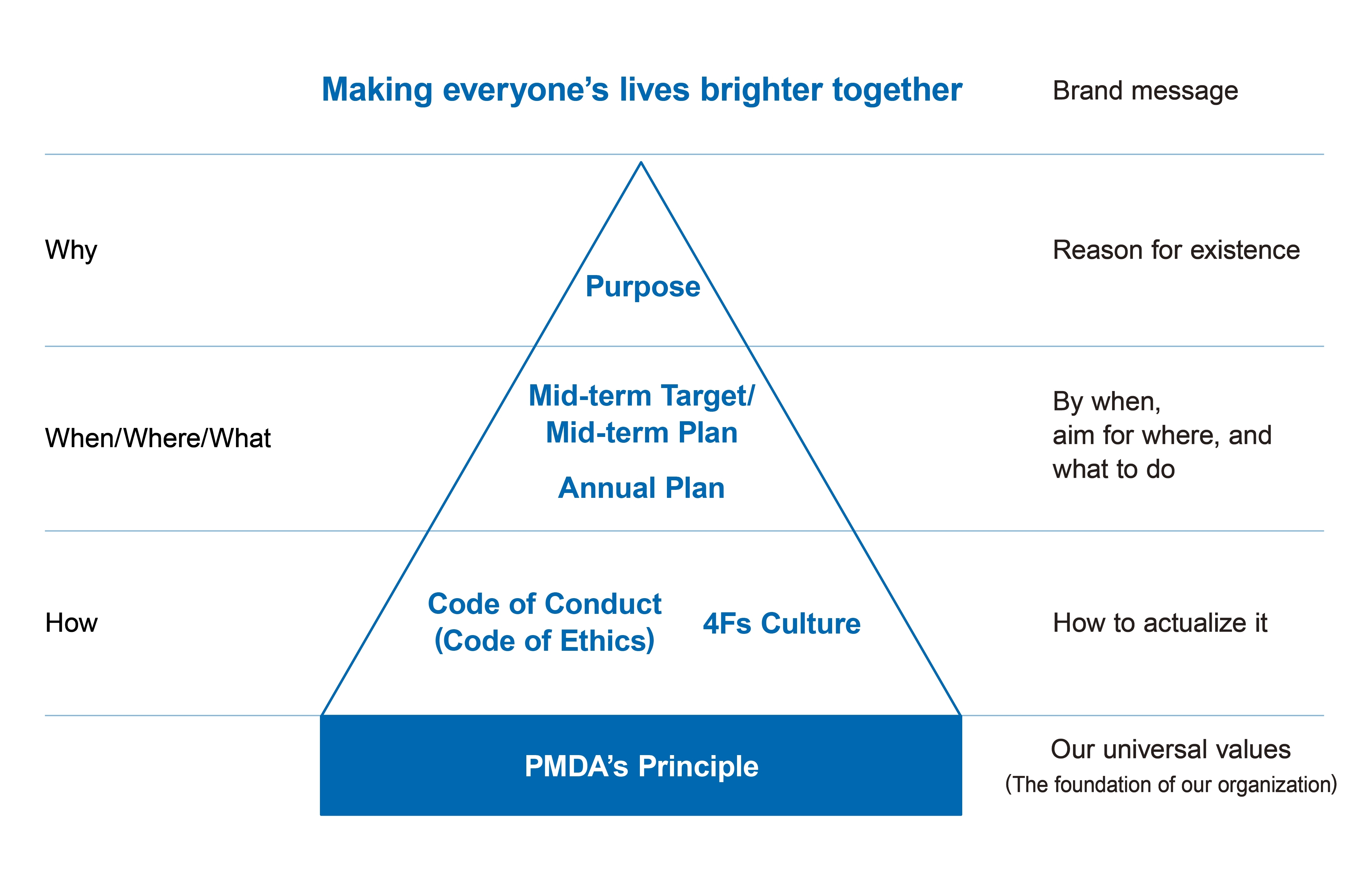
Structure of PMDA Philosophy
PMDA’s Principle
PMDA continues to improve the public health and safety of our nation by reviewing applications for marketing approval of pharmaceuticals and medical devices, conducting safety measures, and providing relief to people who have suffered from adverse drug reactions.
We conduct our mission in accordance with the following principles:
- We pursue the development of medical science while performing our duty with greater transparency based on our mission to protect public health and the lives of our citizens.
- We will be the bridge between the patients and their wishes for faster access to safer and more effective drugs and medical devices.
- We make science-based judgments on quality, safety, and efficacy of medical products by training personnel to have the latest technical knowledge and wisdom in their field of expertise.
- We play an active role within the international community by promoting international harmonization.
- We conduct services in a way that is trusted by the public based on our experiences from the past.
PMDA’s Purpose
The environment surrounding pharmaceuticals, medical devices and regenerative medicines is changing every day.In order to respond flexibly to these changes, the Purpose reflects our wish to mature ourselves further and to create a world where everyone can feel peaceful and can lead vibrant and healthy lives, together with all stakeholders.
Each and every PMDA member works with the Purpose and continues to create "Tomorrow's Normal" together with everyone around the world.
 [97KB]
[97KB]
PMDA’s Values
PMDA Code of Conduct(Code of Ethics)
We conduct our operations with social ethics and values of regulatory science in accordance with the following Code of Conduct in order to put the PMDA philosophy into practice.-
Compliance
We comply with laws and regulations, and act with the highest ethical standards in accordance with social norms. -
Strict Information Management
We strictly manage confidential information, including trade secrets and personal information, which we obtain in the course of our operations. -
Ensure Fairness in the Performance of our duties
We act up to “Honest PMDA” based on high transparency, fairness, sincerity, courtesy, and humility to all stakeholders involved in our operations. -
Enhance the Work Environment
We create a positive work environment and maintain good communications including greetings. -
Health Management
We maintain and manage our own health and are mindful of the health of people around us. -
Prevention of harassment
We respect the dignity and personality of each individual, and we do not tolerate any form of discrimination or harassment. -
Teamwork
We report, communicate, and consult in a timely and appropriate manner, understand others’ standpoint, listen sincerely to other people’s views, and cooperate in our operations. -
Enhance of our operations
We constantly improve our operations, increase efficiency, and aim for higher goals with a positive spirit. -
Proper Management and Use of PMDA Assets
We manage the assets owned by the PMDA so that they are always in a good condition and use them efficiently without mixing public and personal matters.

 [114KB]
[114KB] [112KB]
[112KB]