Nobuo UEMURA 4/27/2011
Foreword
In mid-February, 2010, I moved to the United States Pharmacopeia (USP) secretariat office as a visiting scientist of USP General Chapter team from Japan and a liaison official of MHLW/PMDA ("Ministry of Health, Labour and Welfare" and "Pharmaceuticals and Medical Devices Agency").
This is the seventh report on the USP and FDA issues. However this report is my personal views and opinions, and it does not necessarily represent the formal position of MHLW, PMDA, FDA and USP.
1. Pharmacopeial Forum
The Pharmacopeial Forum (PF) is a bimonthly journal where proposed new and/or revised standards are posted for public review and comment. The contents of the PF are as follows;
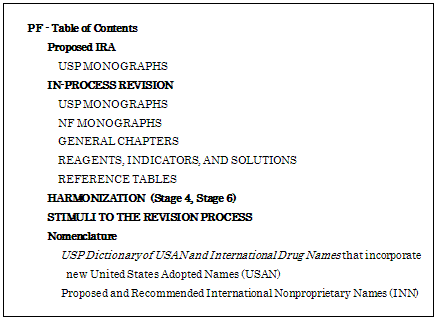
Formerly it was available only to paid subscribers of the United States Pharmacopeia-National Formulary (USP-NF), but the free online PF is available beginning January 3, 2011, by one-time free registration.
Since PF is the platform through which interested parties can submit comments on revisions and additions to the USP-NF before standards become official, USP has opened access to the PF by manufacturers, practitioners, regulators, consumers and other interested parties who may review proposals and offer input during 90 days comment periods.
The free, online-only PF includes only proposals for which USP is seeking public comment, relevant background material, and information about how to utilize PF. The 24 hours online service can access the latest proposed revisions to USP-NF, including in-process revisions, proposed interim revisions announcements, Pharmacopeial Discussion Group Harmonization Proposal (Stage 4,6), Stimuli to the Revision Process, and revision of Nomenclature(USAN,INN). It can search through archived issues of PF from January 2002 to present.
Recent General Chapter proposal in PF (since 2010) is attached.
2. Monograph Modernization Initiative
The resolutions adopted by USP Convention on April 24,2010, says that USP resolves to strengthen its relationship with the FDA, and work with FDA and other public and private stakeholders to explore mechanisms to enable USP to provide and maintain up-to-date national standards for legally marketed drugs and excipients in the United States. (Strengthen USP's Relationship with the U.S.FDA)
In May 2010, USP announced to be actively engaged in efforts to modernize the official USP-NF monographs for small molecules and excipients that utilize outdated technology, have safety and/or environmental concerns, or are missing procedures for key aspects such as impurities. For excipients, a major modernization goal is to replace relatively non-specific identification procedures with specific procedures.
To facilitate the modernization efforts of monographs, USP has established the spreadsheet of monographs in need of modernization, and is seeking proposals of replacement by manufacturers of FDA-approved products, including drug substances and excipients that are known to be used in FDA-approved products, contract laboratories, academic institutions, analytical instrumentation/equipment manufacturers, etc. All submissions include data and other information recommended in the USP Guideline for Submission of Request for Revision to USP-NF. Some modernization proposals may generate new USP Reference Standards.
FDA has established the Monograph Modernization Task Group (MMTG) to focus on the USP monograph modernization initiative, and to identify USP-NF monographs in need of modernization with outdated analytical methods that may make the drug or excipients vulnerable to economically-motivated adulteration (EMA).
In November 2010, FDA provided the list of monographs most in need of modernization, including Acetaminophen, Diphenhydramine, and excipients (Povidone, Crospovidone, Copovidone, Talc). USP continues collaboration with FDA and other stakeholders, collects data, will establish the Expert Panels under the relating Expert Committee, and will hold a workshop on OTC. USP is also conducting laboratory work on Acetaminophen to support possible revisions.
3. USP's verification services
USP verifies the quality, purity, and potency of pharmaceutical ingredients (drug substances and excipients), dietary supplement finished products, dietary supplement ingredients. Products and ingredients that pass all USP verification requirements, including GMP audit, product and ingredient testing, and manufacturing documentation review, are awarded the distinctive USP verified mark.
-USP Verified Pharmaceutical Ingredient Mark
-USP Verified Dietary Supplement Mark
-USP Verified Dietary Supplement Ingredient Mark
The verification services are voluntary available to manufacturers not only in US but also in the world.
Drug substance verification requires;
-Audits of each manufacturing site for compliance with the ICH Q7 GMP Guide for Active Pharmaceutical Ingredients (API),
-Review of manufacturing and QC documents,
-FDA/NIH research partnerships
-Laboratory testing of drug substance samples from selected lots for compliance with labeling and certificate of analysis claims and program requirements
-TPost-verification surveillance testing, audits, and change notification activities conducted to ensure that the drug substance continues to meet program requirements.
In the case of Drug Excipient verification, it requires audits of each manufacturing site for compliance with USP General Chapter <1078> Good Manufacturing Practices for Bulk Pharmaceutical Excipients and International Pharmaceutical Excipients Council's IPEC/PQG GMP Guide for Pharmaceutical Excipients.
4. "Improving Regulation and Regulatory Review - Executive Order"
On January 18, 2011, the President announced the executive order in order to improve regulation and regulatory review. This order reaffirms the principles, structures, and definitions that were established in Executive Order 12866 of September 30, 1993.
It says that US regulatory system must protect public health, welfare, safety, and environment while promoting economic growth, innovation, competitiveness and job creation, and it must be based on the best available science.
As stated in that Executive Order, each agency must: (1) propose or adopt a regulation only upon a reasoned determination that its benefits justify its costs; (2) tailor its regulations to impose the least burden on society, consistent with obtaining regulatory objectives, taking into account the costs of cumulative regulations; (3) select in choosing among alternative regulatory approaches, those approaches that maximize net benefits; (4) to the extent feasible, specify performance objectives, rather than specifying the behavior or manner of compliance that regulated entities must adopt; and (5) identify and assess available alternatives to direct regulation, including providing economic incentives to encourage the desired behavior, such as user fees or marketable permits, or providing information upon which choices can be made by the public. (Sec.1)
Regulations shall be adopted through a process that involves public participation. To the extent feasible and permitted by law, each agency shall afford the public a meaningful opportunity to comment through the Internet on any proposed regulation, with a comment period that should generally be at least 60 days, and shall also provide for both proposed and final rules timely online access to the rulemaking docket on regulations.gov, including relevant scientific and technical findings
Before issuing a notice of proposed rulemaking, each agency shall seek the views of those who are likely to be affected, including those who are likely to benefit from and whose who are potentially subject to such rulemaking. (Sec.2)
In developing regulatory actions and identifying appropriate approaches, each agency shall attempt to promote coordination, simplification, and harmonization across agencies, in order to avoid being redundant, inconsistent or overlapping. (Sec.3)
Where relevant, feasible and consistent with regulatory objectives, and to the extent permitted by law, each agency shall identify and consider regulatory approaches that reduce burdens and maintain flexibility and freedom of choice for the public. (Sec.4)
Each agency shall ensure the objectivity of any scientific and technological information and processes used to support the agency's regulatory actions. (Sec.5)
Agency shall consider how best to promote retrospective analysis of rules that may be outmoded, ineffective, insufficient, or excessively burdensome, and to modify, streamline, expand or repeal them in accordance with what has been learned.
Within 120 days of the date of this order, each agency shall develop and submit to the Office of Information and Regulatory Affairs a preliminary plan, consistent with law and its resources and regulatory priorities, under which the agency will periodically review its existing significant regulations to determine whether any such regulations should be modified, streamlined, expanded or repealed so as to make the agency's regulatory program more effective or less burdensome in achieving the regulatory objectives. (Sec.6)
5. The fiscal year 2011 Appropriations
Since the Republicans, a majority party in the House by the result of the Mid-term election in 2010, requested the spending reduction, the Congress had debated the remainder of FY2011 budget request, while the 6 times Continuing Resolution prevented the shut-down of the Federal Government.
The Continuing Resolution, adopted on March 18, makes $6.1 billion in spending cuts, and it reduces or terminates a total of 25 programs, some of which were terminated or not funded in the President's budget request. The funds of other program were carried over from last year. For example, the bill reduces the "no-year" pandemic influenza funding $276 million, while continuing approximately $65 million in annual flu funding and sufficient carry-over funding.
April 8 (Friday), the dead line when the 3 week continuing resolution would expire, the Democrats and Republicans agreed to determine the FY 2011 budgets reducing $39 billion, and to make additional continuing resolution for one week to prepare the appropriations bill.
The bill of "Appropriation for the Department of Defense and the other departments and agencies of the Government for the fiscal year ending September 30, 2011", was introduced on April 11, approved by both Senate and House on April 14, and signed by the President on April 15. The full-year continuing appropriations would cut about $38 billion in spending.
Under the Act signed by the President, the appropriations for FDA become $3.66 billion, provided under the heading that the $667 million is derived from prescription drug user fees, $62 million is derived from medical device user fees, $450 million is derived from tobacco product user fees, etc.
This funding level is increased from the level of FY2010; $3.28 billion, while taking into consideration the federal government's responsibilities to protect public health and safety in the areas of foods, drugs, medical devices, biologics, etc., but it is reduced about 10 % from the President's initial budget request level; $4.03 billion.
6. President's Fiscal Year 2012 Budget Request
On February 14, 2011, the President sent to Congress his FY2012 budget plan. It lays out a strategy for significant deficit reduction, and it includes a five-year non-security discretionary spending freeze, and down payments on long-term financial reform.
On the other hand, the budget includes investments in the areas critical to future economic growth and job creation; education, innovation, clean energy, and infrastructure. The president mentioned in his budget message that they are increasing investment in research and development that contributes to fields as varied as biomedicine, cyber-security, nano-technology, and advanced manufacturing.
But even in these areas, the budget cuts lower priority programs in order to fund high-priority investments.
7. FDA's FY2012 budget request
The administration's FY 2012 budget request for FDA includes the following priorities;
-Transforming Food Safety and Nutrition
-Advancing Medical Countermeasures (MCM)
-Protecting Patients
-FDA Regulatory Science and Facilities
1)Transforming Food Safety and Nutrition +$218 million / 435 FTE (Full-Time Equivalent)
The Transforming Food Safety and Nutrition Initiative will allow FDA to implement the Food Safety Modernization Act, which was enacted in December 2010. FDA will establish a prevention-focused food safety system, leverage the work of FDA's state and local food safety partners, and empower Americans to make more healthful food choices through nutrition labeling for menu and vending machine items.
2)Advancing Medical Countermeasures +$70 million / 165 FTE
The Advancing Medical Countermeasures (MCM) Initiative strengthens FDA's ability to support the development of MCMs to respond to chemical, biological, radiologic and nuclear (CBRN) threats and to naturally emerging infectious diseases such as pandemic influenza. With this initiative, FDA will enhance the review of MCMs and develop new tools and standards to speed the development of MCMs.
The funds in the MCM Initiative will enable FDA to establish Public Health and Security Action Teams(PHSATs), to establish collaborations with MCM Enterprise partners including Department of Defense, to establish an MCM regulatory science program, to examine the legal framework and regulatory and policy approaches for MCM development and availability to ensure these adequately support emergency preparedness and response.
Advancing Regulatory Science includes;
-animal models for developing and evaluating MCMs,
-clinical biomarkers and immunology to accelerate MCM approval,
-MCM product quality,
-radiation injury protection and response,
-diagnostic platforms for CBRN and other emerging threats,
-health informatics infrastructure to assess MCM safety and efficacy during public health emergencies,
-risk communication strategies for MCM public health emergencies, etc.
3)Protecting Patients Initiative +$124 million / 265 FTE
The Protecting Patients Initiative will allow FDA to develop a pathway for approving follow-on biological products, also known as biosimilars. The initiative also invests in new scientific tools and partnerships to enhance the safety of increasingly complex drugs, medical devices, vaccines and other biological products.
The funds will support
-To establish a regulatory path (policies, criteria, work plan, regulations and guidance documents, information systems, etc.) for biosimilar products.
-To launch an electronic drug registration and listing system to stop illegal drug imports.
-To work closely with foreign drug regulatory authorities to monitor facilities in their countries.
-To expand foreign inspections.
-To expand efforts to protect human subjects in trials across the world.
-To prepare for international collaboration on key medical device safety issues.
-To improve the safety of the blood supply.
-To improve the safety of vaccines.
-To improve the safety of human tissue and cord blood.
-To build a national Medical Device Registry.
-To increase inspections of high-risk medical products
-To expand post-marketing surveillance systems in collaboration with private partners that have access to extensive patient data.
-To reduce medical radiation radiation exposure by developing a National Imaging Dose Registry.
-To reduce the number of childhood poisonings, elderly overdoses, and drug interactions.
-To improve pediatric drug and device safety.
-To improve post-marketing safety in animal drugs.
-To increase the capacity to review generic drugs applications.
-To expand the authority for re-inspection to medical product establishments.
-To conduct FDA import operations associated with surveillance of international courier. etc.
4)FDA Regulatory Science and Facilities +$49 million / 50 FTE
FDA Regulatory Science and Facilities Initiative will allow FDA to establish the scientific infrastructure on the White Oak campus. The initiative also will allow FDA to develop standards for new and emerging technologies, modernize the standards for evaluating products, and accelerate the development of essential medical therapies.
The funds will support;
-To make the CBER-CDER Life Sciences-Biodefense Laboratory
-To strengthen scientific leadership by establishing the Office of the Chief Scientist (OCS).
-To acquire core scientific capacities in emerging technologies (nanotechnology).
-To recruit next generation scientific staff
-To link unique device identifiers (UDIs) to health-related electronic data to create a national medical device registry.
-To foster focused partnerships to transform product development and evaluation sciences, advance personalized medicine, and develop novel diagnostic and medical products.
-To update review standards and provide regulatory pathways for new technologies.
-To refine the guidance for animal biotechnology products.
-To promote development of healthy foods and encourage healthy food choices. etc.
FY 2012 budget request, which covers the period of October 1, 2011, through September 30, 2012, was submitted to the Congress on February 14, 2011, and the Senate and House has already started their committee's hearing on FY 2012 budget request (testimony) in advance of finalizing the appropriations of FY 2011.
