Summary of risk management plan (RMP)
In order to ensure the safety of drugs, it is important to assess measures for appropriate management of the risks of drugs at any time from the development phase to the regulatory review and the post-marketing phase. RMP is a document which shows the consistent risk management of drugs from the development phase to the post-marketing phase. RMP aims for the risks of drugs to be evaluated at regular intervals or in response to the progress of post-marketing surveillance and pharmacovigilance activities to minimize the risks of drugs. It is expected that further enhancements of post-marketing safety measures are ensured by publishing RMP and sharing the information on risk management among healthcare professionals.
What are RMP and RMP materials?
RMP lists already confirmed adverse drug reactions (important identified risks). It also lists adverse events for which a relationship to the administered drug is suspected but not thoroughly verified (important potential risks), and identifies the information considered insufficient (important missing information) for predicting the post-marketing safety of the drug. In addition, to address these risks or missing information, collection of the missing information, examination of risk factors for important identified risks, and causality assessment of important potential risks (the pharmacovigilance activities) or post marketing activities such as information provision for risk minimization (the risk minimization activities) are described. In the risk minimization activities, besides the routine risk minimization activities such as providing information through electronic package inserts, materials (the RMP Materials) may be prepared that are intended for healthcare professionals and patients as additional risk minimization activities. Such materials are prepared for drugs for which additional information provided by materials and other means was considered necessary in the process of the marketing authorization review. Contents of RMP and RMP Materials were confirmed by the PMDA, and the RMP Marking is affixed to these materials to indicate that they are materials prepared in line with RMP.
Utilization of RMP
The PMDA has made efforts to promote utilization of RMP and RMP Materials in clinical settings. RMP and RMP Materials have been posted on the PMDA website, information has been provided through the PMDA Medi-navi, and “Learn about RMP in 3 Minutes,” an RMP made-easy material, as well as “Simple Enough to Start Today! How to Use RMP,” have been prepared and released.
Simple Enough to Start Today! How to Use RMP
The PMDA, in collaboration with the Japanese Society of Drug Informatics and with the intention of enhancing the promotion of utilization of RMP in clinical settings, has developed an e-learning video set titled “Simple Enough to Start Today! How to Use RMP” as an easy-to-understand guide. The set has been available since March 2020 on the PMDA’s YouTube channel and on its website. (Videos are available only in Japanese.)
≪How to view the e-learning videos≫(only on the Japanese site)
The YouTube videos are available directly from the QR code below.

< e-learning content page > (only in Japanese)
https://www.pmda.go.jp/safety/info-services/drugs/items-information/rmp/0002.html
Outline of RMP
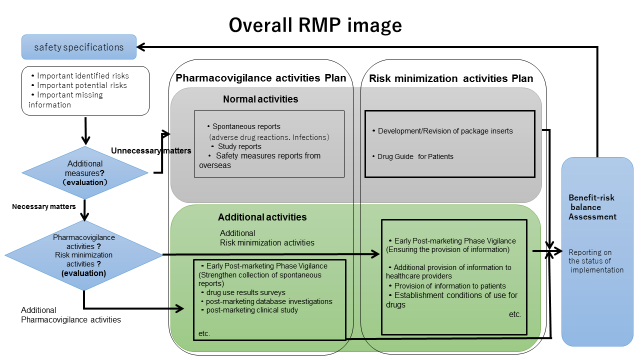
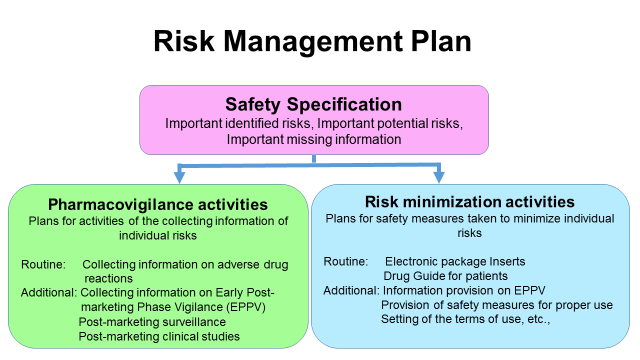
RMP consists of the following three elements for individual drugs: 1) Safety specification 2) Pharmacovigilance activities 3) Risk minimization activities. With regard to pharmacovigilance and risk minimization activities, there are two types of activities such as "routine" and "additional" activities. Routine activities are those to be commonly conducted for all drugs by the marketing authorization holders, specifically collection of information on adverse drug reactions and information provision by package inserts of drugs, etc. Additional activities are those to be conducted individually based on their properties, such as Early Post-marketing Phase Vigilance of new drugs or use-results surveys, post-marketing clinical studies and distributing materials to ensure the proper use of drugs that require caution, etc.
When the PMDA concludes, through the process of an approval review, etc., that additional activities are necessary, the PMDA will publish RMP on its website after the marketing authorization holders submit the RMP including the contents of additional activities to the PMDA.
Details of RMP are shown in the following related notifications and Pharmaceuticals and Medical Devices Safety Information No.378.
| Notification number, etc | Title |
|---|---|
| March 18, 2022 Administrative Notice |
Questions and Answers (Qs and As) on Risk Management Plan [169 KB] |
| March 18, 2022 PSEHB/PED Notification No. 0318-2 PSEHB/PSD Notification No. 0318-1 |
Risk Management Plan templates, instructions and publication [188 KB] |
| December 5, 2017 PSEHB/ELD Notification No.1205-1 PSEHB/SD Notification No.1205-1 (April 26, 2012 Partial amendment of PFSB/ELD Notification No.0426-2 PFSB/SD Notification No.0426-1) |
Partial amendment of "Risk Management Plan templates and instructions" [75.5 KB] (Reference) Risk Management Plan templates and instructions [63.6 KB] |
| April 26, 2012 PFSB/ELD Notification No.0426-2 PFSB/SD Notification No.0426-1 (As partially revised by PFSB/ELD Notification No.0304-1 and PFSB/SD Notification No.0304-1 dated March 4) |
Risk Management Plan templates and instructions for authors [225 KB] |
| March 4, 2013 PFSB/ELD Notification No.0304-1 PFSB/SD Notification No.0304-1 |
Publication of Risk Management Plan [122 KB] Annex [11.2 KB] |
| April 11, 2012 PFSB/ELD Notification No.0411-1 PFSB/ELD Notification No.0411-2 |
Risk Management Plan Guidance [99.5 KB] |
| August 2, 2011 | Risk Management Plan (RMP) Guidance (Draft) [264 KB] |
To healthcare professionals
Publishing RMP will help healthcare professionals to understand what types of risks are known for individual drugs at present. Healthcare professionals are required to know what kinds of surveillance/studies, for which your cooperation is asked, are being conducted as safety measures by the marketing authorization holders.
In order to appropriately use drugs based on the results derived from implementation of post-marketing surveillance/studies scheduled in RMP, the understanding and cooperation of healthcare professionals are necessary.