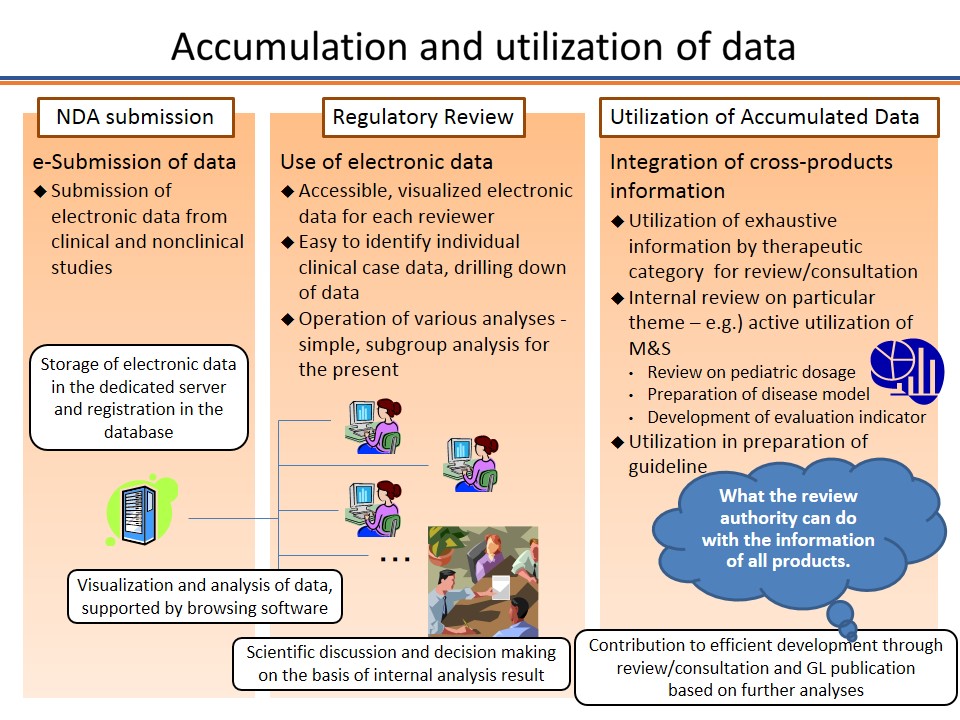
In recent drug development, the use of data-based quantitative information such as those using modeling and simulation (M&S) methods has been proactively promoted in decision-making process.
Under such circumstances, PMDA recognizes the need for accumulating electronic study data, analyzing the data by advanced methods, and making use of the data in the process of its reviews and consultations. The use of such accumulated data is expected to reduce the workload of regulatory submission for sponsors, improve PMDA's evidence-based reviews and consultations, and lead to development of new guidelines, which will eventually result in the rise of the success rate of drug development.
This webpage provides related information about new drug review with electronic data.
Notifications
Revision of Notification on Handling of Submission of Electronic Study Data for New Drug Applications (PSB/PED Notification No. 0408-3, by the Director of Pharmaceutical Evaluation Division, Pharmaceutical Safety Bureau, Ministry of Health, Labour and Welfare, dated April 8, 2024)
Question and Answer Guide Regarding "Notification on Handling of Submission of Electronic Study Data for New Drug Applications” (Administrative Notice of the Pharmaceutical Evaluation Division, Pharmaceutical Safety Bureau, Ministry of Health, Labour and Welfare, dated April 8, 2024)
New Drug Applications Using the Gateway System (PSEHB/PED Notification No. 0401-7, by the Director of the Pharmaceutical Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau, Ministry of Health, Labour and Welfare, dated April 1, 2022)
Revision of Technical Conformance Guide on Electronic Study Data Submissions (PMDA/CPE Notification No. 1345 and PMDA/CRS Notification No. 21, by the Director of Center for Product Evaluation and the Director of Center for RS, Pharmaceuticals and Medical Devices Agency, dated April 8, 2024)
FAQs on Electronic Study Data Submission
This document summarizes inquiries on electronic study data submission received by the PMDA in a Q&A format.
Please utilize these Q&A to resolve problems and questions related to electronic study data submission.
Notice: This is an English version of the following FAQs on electronic study data submission published on April 8, 2024.
Explanation of Electronic Study Data (Form A and Form B)
- Explanation of Electronic Study Data (Form A) [501KB]
- Explanation of Electronic Study Data (Form B) [450KB]
From October 1, 2023 (application date), PMDA does not require to submit "Explanation of Electronic Study Data (Form A)" and "Explanation of Electronic Study Data (Form B)", that describe the contents of electronic study data planned to be submitted to the PMDA, before electronic study data submission for the new drug application.
Please note that Form A and Form B still must be submitted to the PMDA for consultations related to submission of electronic study data for new drug applications.
Data Standards Catalog and Study Data Validation Rules
- Data Standards Catalog (2025-03-14) [24.8KB]
- Study Data Validation Rules
Please note that when submitting electronic study data to the PMDA via the gateway system, the latest version(Version 6.0) of the validation rules is used. However, when applicants submit additional electronic study data after the application, the version of the validation rules at the time of the application will be used.
Although only one version of the validation rule is used for each single application via the gateway system, all versions of these rules — including those no longer open for acceptance — may be used for the validation and the explanation of the results for individual studies performed by an applicant prior to submission. In addition, different versions of the validation rules may be used for SDTM and ADaM datasets of the same study.
- Version 1.0 (2015-11-18) [82.0KB] Acceptable from Oct 1, 2016 to Mar 31, 2021 (application date)
- Version 2.0 (2019-09-27) [97.9KB] Acceptable from Apr 1, 2020 to Mar 31, 2023 (application date)
- Version 3.0 (2021-12-15) [103KB] Acceptable from Jan 1, 2022 to Mar 31, 2025 (application date)
- Version 4.0 (2023-02-28) [112KB] Acceptable from Apr 1, 2023 to Mar 31, 2026 (application date)
- Version 5.0 (2024-03-29) [124KB] Acceptable from Apr 1, 2024 to Mar 31, 2027 (application date)
- Version 6.0 (2025-03-14) [132KB] Acceptable from Apr 1, 2025 (application date)
- CDISC Data Validation Software
The software that PMDA is using is Pinnacle 21 Enterprise 5.1.2, and the engine corresponding to the validation rules are as follows.
- PMDA 1511.6 (Validation Rule Version 1.0)
- PMDA 1810.3 (Validation Rule Version 2.0)
- PMDA 2010.2 (Validation Rule Version 3.0)
- PMDA 2211.1 (Validation Rule Version 4.0)
- PMDA 2311.0 (Validation Rule Version 5.0)
- PMDA 2411.0 (Validation Rule Version 6.0)
On December 15, 2023, PMDA changed the engine from PMDA 2211.0 to PMDA 2211.1 for validation rule version 4.0. This change is intended to resolve an issue of report output and does not change validation results. Therefore, if the validation has been already performed using the previous PMDA 2211.0, there is no need to perform the validation again using the current PMDA 2211.1.
