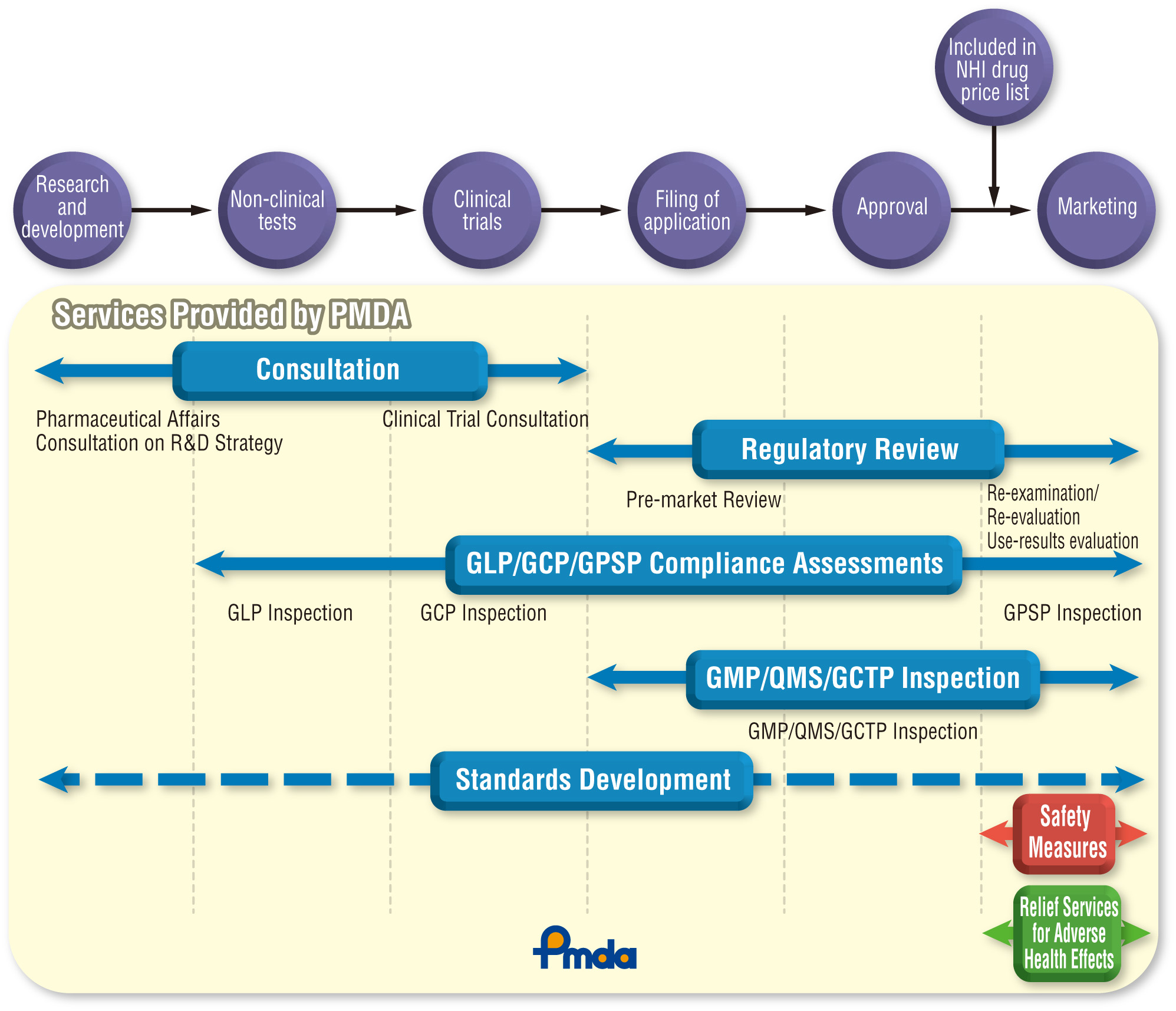
During the review process, PMDA evaluates the quality,efficacy, and safety of drugs, medical devices, and cellular and tissue-based products in light of current scientific and technological standards. In addition, PMDA’s reviews and related services consist of various activities, such as “consultations” providing advice in relation to regulatory submission, GLP/GCP/GPSP inspections to ensure the submitted data are in compliance with the ethical and scientific standards, and GMP/QMS/GCTP inspections to ensure quality management of the manufacturing facility for the product submitted for approval.
Back
Back
-
Regulations & Services of PMDAInformation about Approved Products in JapanSafety InformationAdvanced Efforts
-
Information for approved products in JapanSafety information
-
Development of Drugs, Medical devices, Regenerative medicines and in Vitro diagnosticsInformation for approved products in JapanRegulatory science
-
Regulations and services of PMDAInformation for approved products in JapanProcedures in JapanAdvanced efforts
Back
-
Regulations and Services of PMDAInformation for apporoved products in JapanSafety informationProcedures in Japan
-
Regulations and Services of PMDAInformation for apporoved products in JapanSafety informationProcedures in Japan
-
Reviews and Related ServicesRegulatory ProceduresPost-marketing Safety Measures
-
Reviews and Related ServicesPost-marketing Safety Measures
- Home
- Reviews and Related Services
- Outline
Reviews and Related Services