Consultations
PMDA offers consultations to give guidance and advice on clinical trials of drugs, medical devices, and cellular and tissue-based products as well as on data for regulatory submissions. In clinical trial consultations for new drugs, PMDA checks whether a proposed clinical trial complies with the requirements for regulatory submission, taking into consideration the ethical and scientific aspects and reliability of the clinical trial as well as the safety of trial subjects, and also gives advice to facilitate the improvement of the clinical trial. Starting in FY 2009, PMDA provides prior assessment consultations, in which its reviewers evaluate data on the quality, efficacy, and safety of a product in the pre-submission stage and
the consultation process constitutes part of the review of the product once the application is submitted.
In addition, PMDA has expanded and improved the consultation menu since FY 2007 so as to meet the various requirements for advice on product development and regulatory submission, in such categories as new medical devices and cellular and tissue-based products
that are developed using state-of-the-art technology.
Consultation meeting on R&D or regulatory submission involves consultation fee, while topics to be discussed in such consultations may be clarified free of charge in a Pre-consultation meeting.
Since required procedures, communication, and forms utilized between PMDA are all processed in Japanese, we strongly recommend you to appoint a Japanese Marketing Authorization Holder (MAH) if you are considering entering into Japanese market. Accordingly, consultation meeting should be requested and arranged by intermediary of such MAH.
When you are requesting the free Pre-consultation meeting only, accompanying an interpreter instead of the appointed Japanese MAH may be acceptable.
Consultation fees and other user fees are available from “Fees for reviews and face-to-face consultations, etc.“ page (available only in Japanese).
Please note that fees may be changed without notice.
RS General Consultation/RS Strategy Consultation
Pamphlet: RS general・RS strategy consultation(May 2024 ver.1.0)[2.2MB]
Regulatory Science(RS) General Consultation and Regulatory Science(RS) Strategy Consultation are types of PMDA's consultation services.
In order to achieve realization of innovative drugs, medical devices, and regenerative medical products, PMDA launched the RS General Consultation and the RS Strategy Consultation, mainly for universities, research institutions, and venture companies that possess promising “seed-stage” researches or technologies. In the consultations, advice will be provided on the tests needed in the early product development stage and the necessary clinical trials.
The scope of these consultations is from the final stage of selection of candidates for development to the initial stage of clinical development (proof of concept (POC) studies (Phase IIa)).
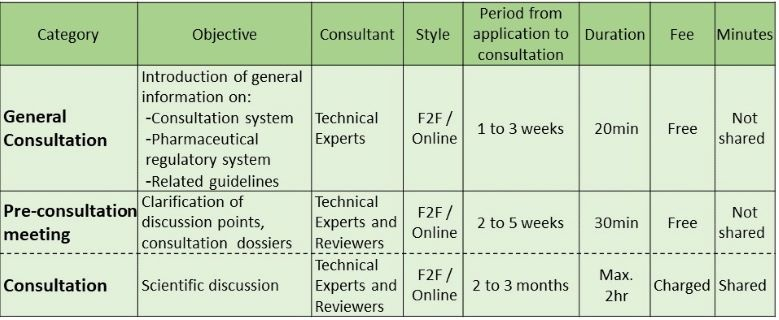
There are a few types of RS related consultations as shown in the below figure.
- RS General Consultation
- Pre-consultation meeting for RS Strategy Consultation
- RS Strategy Consultation
 [143KB]
[143KB]
The lower consultation fee will apply for RS Strategy Consultation if all the following prerequisites are fulfilled. See "RS Strategy Consultation"
For the RS General Consultaion and the RS Strategy Consultation, please contact us through following e-mail address;
rs-contact[at]pmda.go.jp
Note:
- Please replace [at] with @ when you send an e-mail.
- It will normally take about 5 working days to answer.
Before sending your inquiry, please read following points carefully.
- Please check our Frequently Asked Questions (FAQ) if your current inquiry is already described.
- Please check the following information pages.
- PMDA does not control the followings. Please refer to the Contact List and contact the respective authorities or organizations on these matters directly.
- - the pricing and reimbursement of medical products
- - patents on medical products
- - food products including supplements
- - cosmetics
- - Japanese Pharmaceutical Excipients (JPE) and Japanese Pharmaceutical Codex (JPC)
- - medical products for animal use
- - distribution and import of medical products
- - marketing license for medical products
- - license for wholesale distribution
Prerequisites for fee reduction in RS Strategy Consultation
In principle, all of the following prerequisites have to be fulfilled.
(Venture companies)
- An SME (i.e., the number of employees is 300 or less or the company’s capital is JPY 300MM or less.
- Another corporate body does not hold shares or capital contributions equivalent to one half or more of the total number of shares or the total amount of contributions.
- Two or more corporate bodies do not hold shares or capital contributions equivalent to two thirds or more of the total number of shares or the total amount of contributions.
- Net profit is not recorded or is recorded without business revenue in the previous fiscal year.
