Drugs Reviews
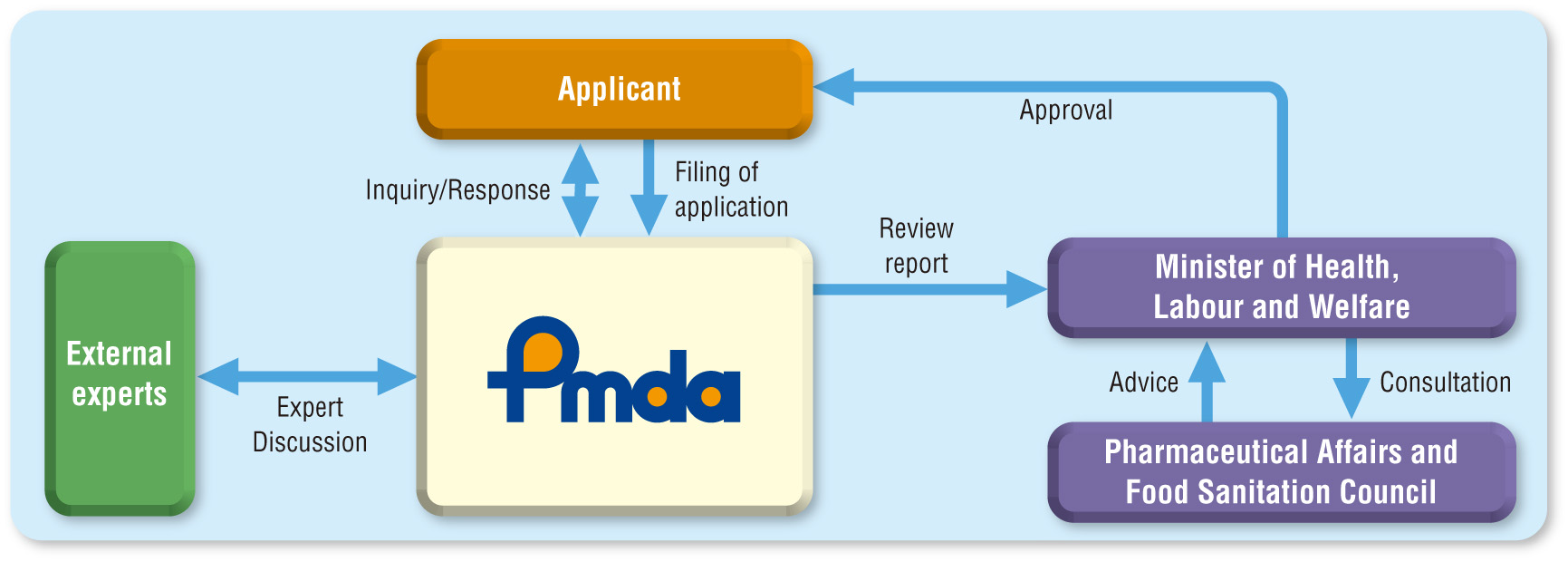
In the review of drug applications, PMDA reviewers, who have degrees in pharmaceutical science, medicine, veterinary medicine, physical science, biostatistics, or other specialties, form a team to evaluate the quality, pharmacology, pharmacokinetics, toxicology, clinical implications, and biostatistics regarding the particular drug product under review. During the review process, the reviewers exchange opinions with external experts (Expert Discussions) to ensure that more effective reviews are conducted by making use of their advanced expertise. In addition, PMDA participates in the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and has actively incorporated the guidelines agreed upon at ICH into its drug reviews.
PMDA strives to speed up the review process by setting target review times, while clarifying the standards for review by publishing the basic considerations for reviewers on its website.
PMDA’s drug reviews encompass not only new drugs but also generic drugs, OTC drugs / “behind-the-counter (BTC)” drugs (requiring pharmacists’ advice) that can be purchased at pharmacies without a doctor’s prescription, and quasi-drugs. PMDA also conducts re-examinations and re-evaluations of approved drug products.
In FY 2013, a total of 7118 drugs were approved, of which 4008 were prescription drugs (including 504 new drugs), 916 were OTC drugs / BTC drugs, 166 were in vitro diagnostics, and 2028 were quasi-drugs.
| Priority review products refer to orphan drugs (expected to be used by less than 50,000 patients) and products designated for priority review by the Ministry of Health, Labour and Welfare in consideration of their clinical usefulness and the seriousness of the diseases for which they are indicated. |
Review Process for Drug or Medical Device Application
Medical Devices Reviews
Medical devices cover a wide range of products, from adhesive bandages and forceps to MRI and pacemakers, which are characterized by a variety of usage patterns and different levels of risk. Among these products, highrisk medical devices, including artificial hearts and pacemakers, are evaluated by PMDA. As with drug reviews, PMDA has set target review times for medical devices and is working hard to achieve these targets through various efforts, such as increasing the number of reviewers.
In evaluating medical devices, in addition to reviewers who possess expertise in medical engineering, biological engineering, and biomaterials, specialists with degrees in medicine, dentistry, pharmaceutical science, and other fields are involved in non-clinical, clinical, and biostatistical evaluations. During the review process, the reviewers exchange opinions with external experts (Expert Discussions) to enable more highly specialized reviews.
To promote international regulatory harmonization, PMDA participates in the International Medical Device Regulators Forum (IMDRF) and other meetings, while improving its review system by actively incorporating the topics discussed at international conferences as well as standards such as those of the International Organization for Standardization (ISO).
Regenerative Medical Products Reviews
Regenerative medical products have been newly defined by the Pharmaceuticals and Medical Devices (PMD) Act that was promulgated on November 27, 2013.
Regenerative medical products in the PMD Act are defined as below.
Regenerative Medical Products refer to:
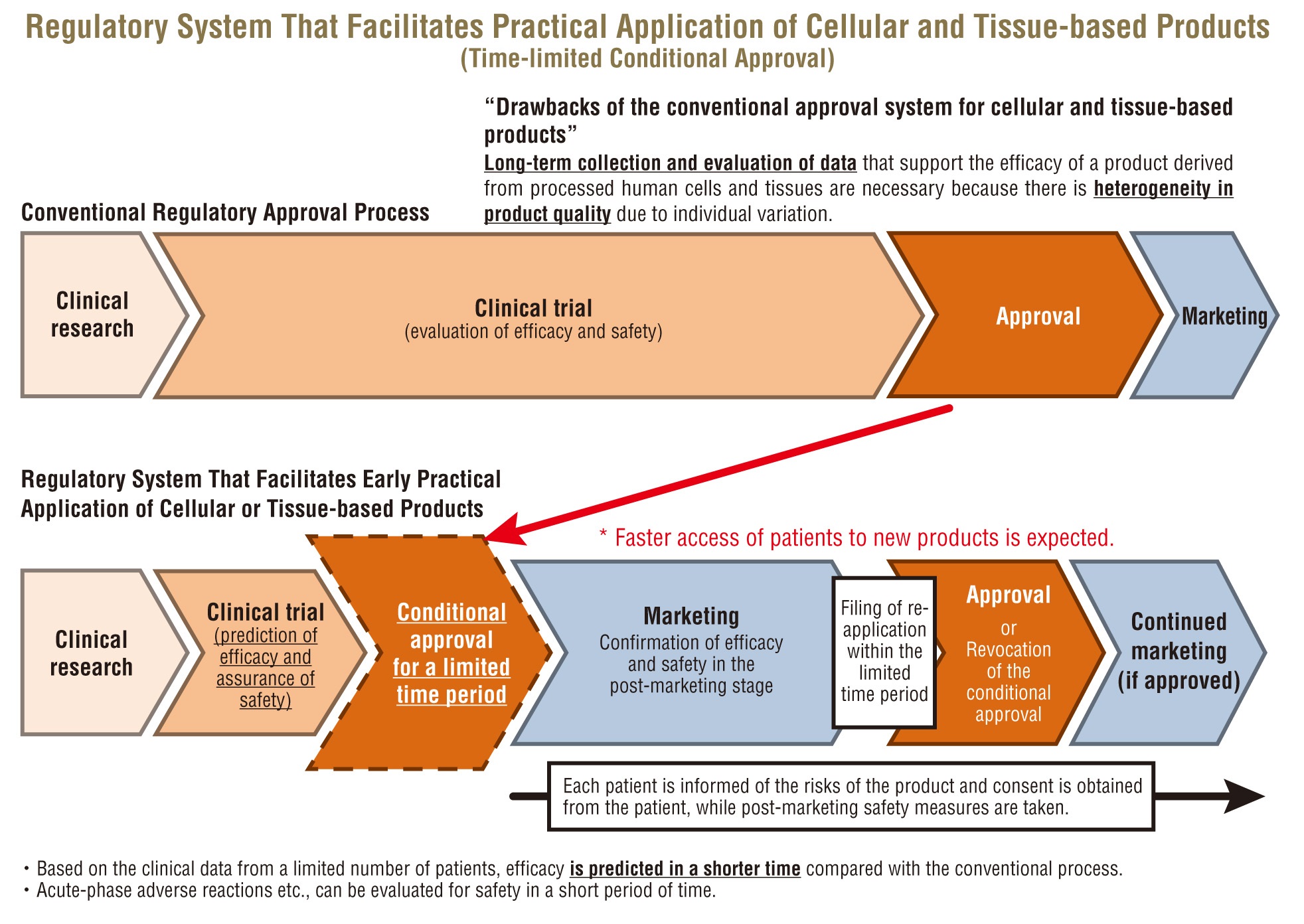
* Since these products are all derived from processed living cells/tissues, the products are characterized by their varied quality and in that their efficacy is difficult to be confirmed in some cases. |
Regeneraive medical products have properties different from those of conventional drugs and medical devices. For example, the quality of a product derived from living human or animal cells/tissues may vary. Even such a product can be swiftly approved with conditions for a limited time period if its efficacy is predicted and its safety is assured, since “the time-limited conditional approval system” has been introduced under the new legislation.
PMDA has established an operation system capable of appropriately and promptly responding to the newly introduced regulatory approval system through various approaches such as enhancing expertise of its reviewers.
For more detail, please visit our Regenerative Medical Products Information site.