
Click here to see the Promotion Flyer[214KB]
Introduction
The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to invite regulators to the “PMDA-ATC Medical Devices Seminar 2023". Officials from many regulatory authorities who are engaged in the review of medical devices are welcomed. This seminar will be held in-person at the PMDA Office in Tokyo from December 5 to 7, 2023.
This seminar will cover implementation and adaptation of Japanese medical device regulation based on GHTF/IMDRF documents, such as IVD, AI-based medical devices, review of high risk medical devices, Software as a Medical Device (SaMD), etc. Small group discussions among participants on case studies are planned as well.
Trainees will have chances to know recent practical application of international standards in PMDA that may enhance the regulatory systems for medical devices within your own regulatory framework.
Please be noted that this seminar is NOT an “APEC Center of Excellence (CoE) workshop” which follows the core curriculum of the RHSC Medical Devices Priority Work Area (PWA). An announcement of the APEC CoE Medical Devices workshop 2023 will be released separately.
Key Objectives of This Seminar
- To learn regulations of specific medical devices, especially in the following fields.
- IVD medical devices
- AI-based medical devices
- High risk (Classes C and D) medical devices
- Software as a Medical Device (SaMD)
- To obtain up-to-date information about some specific medical devices of high interest.
- To utilize the knowledge learned from the webinar to enhance the regulatory system in the participant’s own organization.
Who should apply
Regulators ONLY (Beginner to Intermediate level)
- Employees of regulatory authorities, or other agencies/institutes closely related thereto, who are currently engaged in the review of medical devices and in vitro diagnostics.
- Basic knowledge of the regulations pertaining to the medical devices product review in your organization is required.
- English will be used in the lectures and discussions during the seminar. Participants need an ability to readily communicate in English.
Date / Time
- December 5 (Tuesday), 2023 : 10:00 to 16:00 JST (UTC+9)
- December 6 (Wednesday), 2023 : 10:00 to 17:20 JST (UTC+9)
- December 7 (Thursday), 2023 : 10:00 to 12:40 JST (UTC+9)
Program (subject to change)
See the attached program[217KB]
Application (Registration closed)
Number of participants: approx. 30
Close of registration application : September 12, 2023
To apply for the seminar, please make sure to complete all the necessary items on the application form, including the signature by the applicant's head of the organization, before the closing date.
Click the link to apply: https://www12.webcas.net/form/pub/pmda-atc/md2023_pmdaws
- If applications exceed the upper limit of 30, selection of the participants will take place at the discretion of PMDA. Participants will be selected from the person who have completed the PMDA-ATC Medical Devices Review E-learning Course on a priority basis.
Click the link to apply: https://www12.webcas.net/form/pub/pmda-atc/e-learning02
- Confirmation of registration and additional information will be sent to the approved participants after the closing date.
Others
- Materials will be provided electronically. Participants are recommended to bring their own personal computer to access these materials when needed.
- There may be COVID 19-related border measures to visit Japan and return to your country. Please confirm the current measures at your end.
Fee
There is no registration fee for this seminar.
- Information on travel and hotel reservation assistance will be provided to the approved participants.
- International Travel insurance is not covered by PMDA, but PMDA strongly recommends participants consider obtaining it on your own.
Seminar Location
Pharmaceuticals and Medical Devices Agency (PMDA):
Shin-kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013, Japan
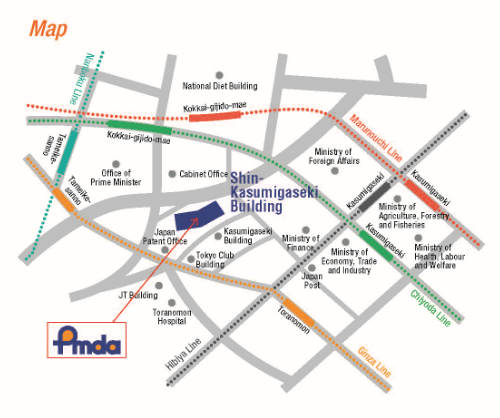
Please use public transportation. Nearest Tokyo Metro Subway Stations:
5 minutes' walk from Exit 11 of Toranomon Station on the Ginza Line
8 minutes' walk from Exit 3 of Kokkai-gijido-mae Station, on the Marunouchi Line, Chiyoda Line
8 minutes' walk from Exit A13 of Kasumigaseki Station, on Marunouchi Line, Chiyoda Line, Hibiya Line
10 minutes' walk from Exit 8 of Tameike-sanno Station, on the Ginza Line, Nanboku Line
Contact Us
For more information, please contact:
Secretariat, PMDA Asia Training Center
E-mail: PMDA-ATC[at]pmda.go.jp
Division of Training Center Management
Office of International Programs
Pharmaceuticals and Medical Devices Agency
Shin-Kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013, Japan
(Note: For the purpose of security, @ in the E-mail address is replaced with [at]. Please replace [at] with @ when you send an E-mail.)
