
Click here to see the Promotion Flyer[143KB]
Introduction
The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to announce the “PMDA-ATC with National Cancer Center MRCT Seminar 2024” for officials of new drug application reviewers from overseas regulatory authorities. The seminar is offered by the Asia Training Center for Pharmaceuticals and Medical Devices Regulatory Affairs (PMDA-ATC), with the collaboration of the Clinical Research Support Office of the National Cancer Center Japan (NCC). The seminar will be held in person at the PMDA Office in Tokyo from January 23 to 26, 2024.
This seminar is held as the workshop of Asia-Pacific Economic Cooperation, Regulatory Harmonization Steering Committee (RHSC) Center of Excellence.
The seminar aims to promote drug development and convergence of regulations by sharing accumulated experiences of Japan, such as protocol designing and planning of MRCT, clinical operation and clinical data evaluation, and discussing clinical trial networking with participants from around the world. The seminar will be led not only by PMDA reviewers and NCC experts but also by representatives from academia and industry, allowing participants to think and discuss from multiple perspectives.
Key Objectives of This Seminar
- To learn the current situation surrounding MRCTs.
- To obtain an opportunity to discuss how to make regulatory decisions based on specific cases.
- To build a skilled human capacity in regulatory science to promote and facilitate MRCTs.
- To enhance the regulatory and clinical cooperation in the APEC region on the evaluation and regulation of MRCTs.
- To support for establishment and maintenance of clinical trial sites in Asian region
Who should apply
Regulators ONLY (Intermediate level)
- Senior clinical reviewers with at least 3 years of hands-on experience in the assessment of clinical trial applications, benefit-risk profiles for market approvals.
- English will be used in the lectures and discussions during the seminar. Participants need an ability to readily communicate in English.
- Regulators from non-APEC economies are also welcomed.
- All participants are encouraged to take and complete the PMDA-ATC E-learning Course of “MRCT” prior to attending the live sessions as the pre-requirement.
Date / Time
- January 23 (Tuesday), 2024 : 10:00 to 16:15 JST (UTC+9)
- January 24 (Wednesday), 2024 : 10:00 to 16:00 JST (UTC+9)
- January 25 (Thursday), 2024 : 10:00 to 16:00 JST (UTC+9)
- January 26 (Friday), 2024 : 10:00 to 12:00 JST (UTC+9)
Program (subject to change)
See the attached program[280KB]
Application (Registration closed)
Number of participants: approx. 30
Close of registration application: October 24, 2023
To apply for the seminar, please make sure to complete all the necessary items on the application form, including the signature by the applicant's head of the organization, before the closing date.
Click the link to apply: https://www12.webcas.net/form/pub/pmda-atc/mrct2024
- If applications exceed the upper limit of 30, selection of the participants will take place at the discretion of PMDA. Participants will be selected from the person who have completed the PMDA-ATC MRCT E-learning Course on a priority basis.
Click the link to apply: https://www12.webcas.net/form/pub/pmda-atc/e-learning02
- Confirmation of registration and additional information will be sent to the approved participants after the closing date.
Others
- Materials will be provided electronically. Participants are recommended to bring their own personal computer to access these materials when needed.
Fee
There is no registration fee for this seminar.
- Information on travel and hotel reservation assistance will be announced to the approved participants.
- International travel insurance is not covered by PMDA, but PMDA strongly recommends participants to consider obtaining it on your own.
Seminar Location
Pharmaceuticals and Medical Devices Agency (PMDA):
Shin-kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013, Japan
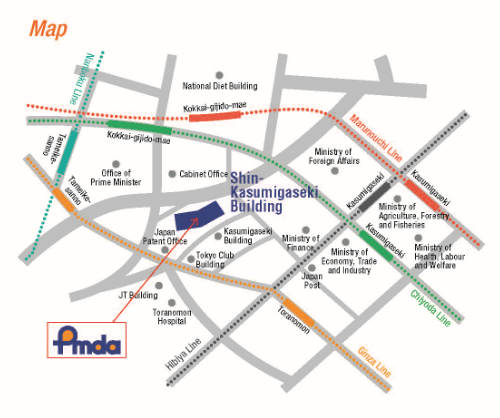
Please use public transportation. Nearest Tokyo Metro Subway Stations:
5 minutes' walk from Exit 11 of Toranomon Station on the Ginza Line
8 minutes' walk from Exit 3 of Kokkai-gijido-mae Station, on the Marunouchi Line, Chiyoda Line
8 minutes' walk from Exit A13 of Kasumigaseki Station, on Marunouchi Line, Chiyoda Line, Hibiya Line
10 minutes' walk from Exit 8 of Tameike-sanno Station, on the Ginza Line, Nanboku Line
Contact Us
For more information, please contact:
Secretariat, PMDA Asia Training Center
E-mail: PMDA-ATC[at]pmda.go.jp
Asia Training Center for Pharmaceuticals and Medical Devices Regulatory Affairs, Pharmaceuticals and Medical Devices Agency
Shin-Kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013, Japan
(Note: For the purpose of security, @ in the E-mail address is replaced with [at]. Please replace [at] with @ when you send an E-mail.)
