Introduction
The Pharmaceuticals and Medical Devices Agency (PMDA) are pleased to announce the “PMDA-ATC Pharmaceuticals Review Seminar 2024 for PPWG member states” for reviewers of ASEAN PPWG member states. The seminar will be held in person at the PMDA Office in Tokyo from June 11 to 14, 2024.
This seminar focuses on new drug review as practical training. It will cover topics relating to Japanese regulation, points to consider in evaluation of new drug review, structure and content of PMDA review report, Risk Management Plan (RMP), and case studies using PMDA approved product.
Key Objectives of This Seminar
- To strengthen data evaluation skill of reviewers of ASEAN regulatory authorities.
- To support ASEAN Joint Assessment (JA) and the bilateral reliance scheme.
- To contribute to national assessment of drug review conducted by each authority.
Who should apply
Regulators of ASEAN PPWG member states ONLY (Intermediate or higher level)
- Working experience of 1) pharmaceuticals review for over 4 years or 2) ASEAN JA.
- English will be used in the lectures and discussions during the seminar. Participants need an ability to readily communicate in English.
- All participants are recommended to take self-learning lecture videos prior to attending the seminar.
Date / Time
June 11 (Tuesday), 2024 10:00 JST (UTC+9) to June 14 (Friday), 2024 16:30 JST (UTC+9)
Program
See the attached program [115KB]
Fee
There is no registration fee for this seminar.
- Information on travel and hotel reservation assistance will be announced to the approved participants.
- International travel insurance is not covered by PMDA, but PMDA strongly recommends participants to consider obtaining it on your own.
Seminar Location
Pharmaceuticals and Medical Devices Agency (PMDA):
Shin-kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013, Japan
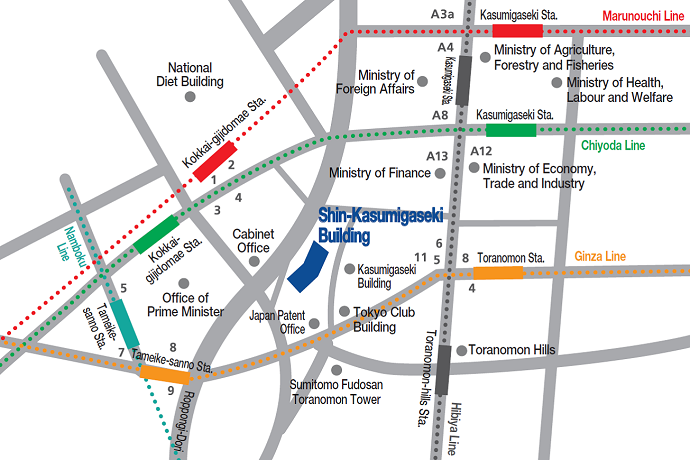
Please use public transportation. Nearest Tokyo Metro Subway Stations:
5 minutes’ walk from Exit 11 of Toranomon Station on the Ginza Line
8 minutes’ walk from Exit 3 of Kokkai-gijido-mae Station, on the Marunouchi Line, Chiyoda Line
8 minutes’ walk from Exit A13 of Kasumigaseki Station, on the Marunouchi Line, Chiyoda Line, Hibiya Line
10 minutes’ walk from Exit 8 of Tameike-sanno Station, on the Ginza Line, Namboku Line
Contact Us
For more information, please contact:
Secretariat, PMDA Asia Training Center
E-mail: PMDA-ATC[at]pmda.go.jp
Asia Training Center for Pharmaceuticals and Medical Devices Regulatory Affairs Pharmaceuticals and Medical Devices Agency
Shin-Kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan
(Note: For the purpose of security, @ in the e-mail addresses are replaced with [at]. Please replace [at] with @ when you send an e-mail.)
