

Click here to see the Promotion Flyer [445KB]
Introduction
The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to announce that we will be organizing the “PMDA-ATC GMP Inspection Seminar 2025” for overseas regulatory authority officials who are engaged in GMP inspection of pharmaceuticals.
The seminar will be held IN PERSON at the PMDA Office in Tokyo from September 17 to 19, 2025.
The seminar is aligned with PIC/S training content, it could be a good way to familiarize oneself with their standards. It could give participants a foundation to prepare for the 2025 PIC/S Seminar in HKSAR as well.
Kindly note that this is not open to industry or non-regulatory authorities. We welcome participation from many regulatory authorities.
Key Objectives of this Seminar
- To assist participants in developing a good understanding of regulations on GMP Inspections, especially in view of the following:
- Advanced technologies in pharmaceutical manufacturing
- GMP inspection Reliance in Asia and possibly other multi-regional settings
- To enhance the inspection capacity of participating authorities, by utilizing knowledge from the seminar, thus improving the GMP system of the participant’s own country.
Who should apply
Regulators ONLY (Intermediate level)
- Staff of regulatory authorities with solid working experience of onsite GMP inspections, e.g. having led a minimum of 5 GMP inspections at different manufacturing sites of pharmaceutical products.
- Over 3 years' experience working in a GMP regulatory department and intermediate knowledge of GMP regulations are required.
- English will be used in the lectures and discussions. Participants need an ability to readily communicate in English.
- All participants are recommended to view designated material before participating in the seminar.
Date / Time
- September 17 (Wednesday), 2025 : 9:30 to 16:15 JST (UTC+9)
- September 18 (Thursday), 2025 : 10:00 to 16:30 JST (UTC+9)
- September 19 (Friday), 2025 : 10:00 to 12:00 JST (UTC+9)
Program (subject to change)
Participants/Application (Registration Closed)
Number of participants: approx. 30
Close of registration application: June 18, 2025
To apply for the seminar, please make sure to complete all the necessary items on the application form, including the signature by the applicant's head of the organization, before the closing date.
Click the link to apply: (https://atcform.pmda.go.jp/servlet/front?id=53&p=1&m=1)
- If eligible applications exceed 30, participants will be finalized at the discretion of PMDA.
- Confirmation of registration and additional information will be sent to the finalized participants after the closing date.
Others
- Course material will be provided electronically. Participants are recommended to bring their own personal computer to access these when needed.
Fee
There is no registration fee for this seminar.
- Details on travel and hotel reservation assistance will reach participants. Further information available at: https://www.pmda.go.jp/english/int-activities/training-center/0023.html
- International travel insurance is not covered by PMDA, but PMDA strongly recommends all participants to purchase a plan.
Seminar Venue
Pharmaceuticals and Medical Devices Agency (PMDA):
Shin-kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013, Japan
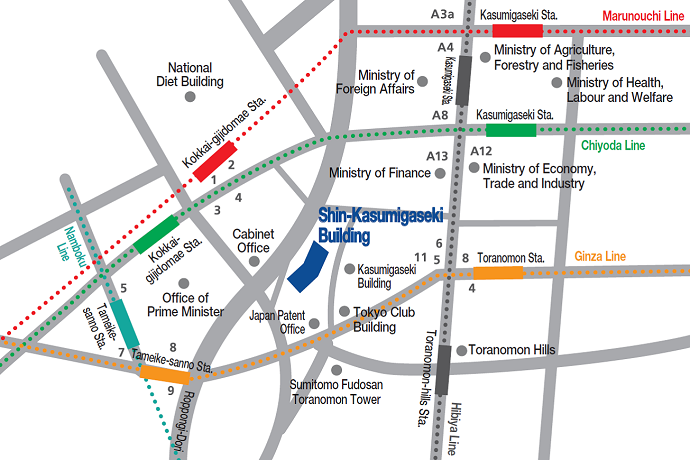
Please use public transportation. Nearest Tokyo Metro Subway Stations:
- Toranomon - Ginza Line (G07) 5 min from Exit 11
- Kokkai-gijido-mae - Marunouchi Line, Chiyoda Line (M14, C07) 8 min from Exit 3
- Kasumigaseki - Marunouchi Line, Chiyoda Line, Hibiya Line (M15, C08, H07) 8 min from Exit A13
- Tameike-sanno - Ginza Line, Namboku Line (G06, N06) 10 min from Exit 8
Contact Us
For more information, please contact:
Secretariat, PMDA Asia Training Center
E-mail: PMDA-ATC[at]pmda.go.jp
Asia Training Center for Pharmaceuticals and Medical Devices Regulatory Affairs Pharmaceuticals and Medical Devices Agency
Shin-Kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan
(Note: For the purpose of security, [at] in the e-mail addresses are replaced with @. Please replace [at] with @ when you send an e-mail.)
