About MDSAP
The Medical Device Single Audit Program (MDSAP) is intended to allow MDSAP Auditing Organizations to conduct a single audit of a medical device manufacturer that will satisfy the relevant requirements of the medical device regulatory authorities participating in the program.
Japan's Ministry of Health, Labour and Welfare, and the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has been participating to the program since June 2015.
International partners that are participating in the MDSAP include:
- Therapeutic Goods Administration of Australia,
- Brazil's Agência Nacional de Vigilância Sanitária,
- Health Canada, and
- U.S. Food and Drug Administration.
- The World Health Organization (WHO) Prequalification of In Vitro Diagnostics (IVDs) Programme and the European Union (EU) are Official Observers
Acceptance of MDSAP Audit Reports
MDSAP audit reports may be used as a way to demonstrate conformance to Japanese medical device QMS requirements (see details).
PMDA has been accepting MDSAP audit reports since 2016. The following chart illustrates the results of the acceptance from 2017 to 2024.
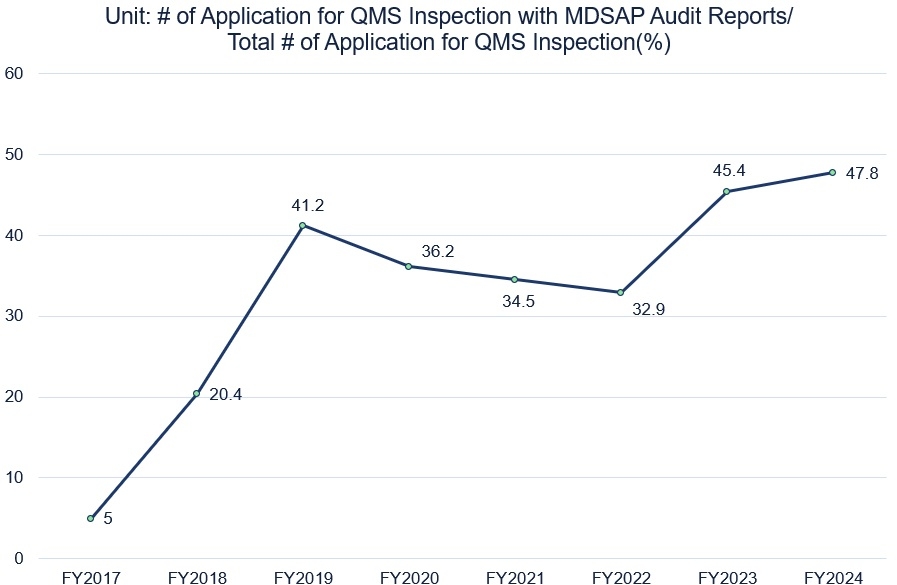
PMDA acceptance of MDSAP audit reports from FY2017 to FY2024
As a result of the discussion between stakeholders, the Japanese Regulatory Authorities, MHLW/PMDA, have decided to formally use MDSAP audit reports as one of the way to confirm comformity to the regulatory requirements.
