Overview
- The Act on the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms (Act No. 97 of 2003) (The Cartagena Act) aims to ensure biodiversity through international cooperation. The Cartagena Protocol on Biosafety to the Convention on Biological Diversity is an international agreement that aims to ensure the safe handling, transport, and use of living modified organisms (LMO) resulting from modern biotechnology that may adversely affect biological diversity and pose threat to human health. It came into effect in 2014 as a law to properly operate the Cartagena Protocol on Biosafety to the Convention on Biological Diversity by implementing measures that regulate the use of LMO, which are similar to genetically modified organisms(GMO).
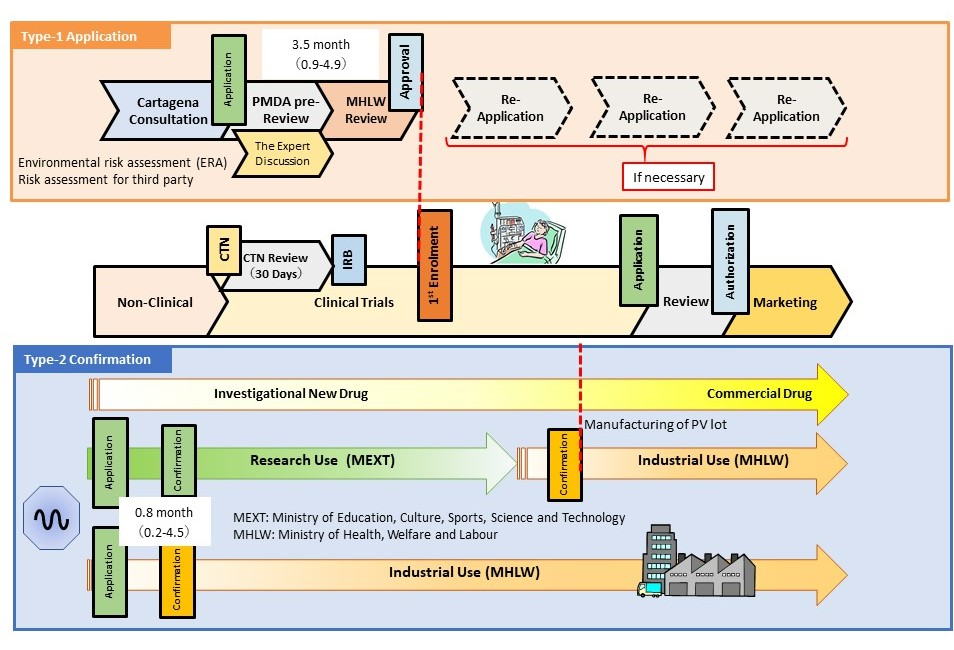
- The Cartagena Act is a law concerning the use of LMO/GMO and covers the use of LMO/GMO as medical products in Japan. “Approval” by the competent ministers (MHLW and Minister of the Environment) is required when attempting type-1 use (no measures are taken to prevent the release of LMO/GMO to the environment). In addition, “confirmation” by the competent ministers is required when attempting type-2 use for industrial purposes (intended for use by preventing the diffusion of LMO/GMO into the air, water, or soil outside facilities, equipment, and other structures indicated in Article 2, Paragraph 6 of the Act) . When developing LMO/GMO, such as viral vectors, confirmation with the MHLW regarding type-2 use is required before starting the manufacture. It is necessary to obtain approval from the MHLW regarding type-1 use at a medical institution before enrolling the first patient in the clinical trial.
- The PMDA conducted reviews of type-1 use and type 2 use under the Cartagena Act.
Definition of LMO/GMO in the Cartagena Act.
- LMO shall mean an organism that possesses nucleic acid, or a replicated product thereof, obtained through use of the any of the following technologies.
- (i) Those technologies as stipulated in the ordinance of the competent ministries, for the processing of nucleic acid extracellularly
- (ii) Those technologies, as stipulated in the ordinance of the competent ministries, for fusing of the cells of organisms belonging to different taxonomical families
- Included in LMO/GMO of the Act
- Genetically modified bacteria
- Genetically modified animals
- Genetically modified plants
- Genetically modified viruses (including vaccine strains)
- Not included in LMO/GMO of the Act
- Attenuated viruses for vaccines
- Genetically modified animal cells (CHO cells, etc.)
- Gene edited animals without foreign nucleic acid fragments
- Self-cloning/natural occurrence
Type-1 Use approval and Type-2 Use confirmation
- Type-1 (Approval)
<Deliberate release>
Using GMOs under the rules to minimize the environmental impacts.
Point for review
Environmental Risk Assessment+Risk Assessment for third party
Examples- Administration of viral vectors for in vivo gene therapy
- (Out of scope: Administration of viral vector-free CAR-T cells)
- Type-2 (Confirmation)
<Containment Use>
Using GMOs under the measure to prevent GMOs from diffusing into the environment.
Point for review
Appropriate uses depending on the biosafety levels of GMOs
Examples- Manufacturing of viral vectors for in vivo gene therapy
- Manufacturing of CAR-T cells using viral vectors
Cartagena Act in Product Development
Comparison of GMO regulations (Type-1 Use regulation, Gene therapy)
Operational improvement and recent review performance
- The PMDA has set a new consultation menu related to the Cartagena Act. This consultation is conducted prior to submission of the application for type-1 use, or submission of the application for confirmation of type-2 use under the Cartagena Act. The consultation intends to provide guidance and advice regarding the sufficiency of data for submission, and appropriateness of description for each individual LMO/GMO and for matters specified in regulations on type-1 use or type 2-use. In contrast, the voluntary PMDA action, known as the “draft application checking process”, was eliminated due to the replacement of official consultations in 2022. Several other improvements (application for partial change, publishing of the instructions for environmental risk assessment, and specific environmental risk assessment for adeno-associated viruses) have been implemented, making the administrative process of the Cartagena Act rapid and predictable.
- Administrative Review Results (2019-)
| Type-1 | FY2019 (8 cases) |
FY2020 (6 cases) |
FY2021 (8 cases) |
FY2022 (15 cases) |
FY2023 (5 cases) |
FY2024 (9 cases) |
|---|---|---|---|---|---|---|
| Review Time Median (Min-Max) [months] |
4.7 (2.6-6.6) |
4.2 (3.6-6.3) |
3.2 (0.9-4.3) |
3.3 (1.3-5.3) |
2.8 (1.1-4.0) |
3.5 (2.0-4.5) |
| Total Time (Note) Median (Min-Max) [months] |
5.9 (3.2-9.9) |
6.6 (5.1-10.5) |
3.5 (0.9-4.9) |
5.0 (1.3-7.5) |
3.1 (1.2-7.1) |
4.2 (2.8-7.2) |
(Note) Reference Value
| Type-2 | FY2019 (28 cases) |
FY2020 (45 cases) |
FY2021 (50 cases) |
FY2022 (18 cases) |
FY2023 (35 cases) |
FY2024 (10 cases) |
|---|---|---|---|---|---|---|
| Review Time Median (Min-Max) [months] |
0.9 (0.3-1.2) |
1.1 (0.2-2.1) |
0.8 (0.2-2.9) |
1.3 (0.6-2.1) |
1.4 (0.2-1.5) |
1.1 (0.5-1.8) |
| Total Time (Note) Median (Min-Max) [months] |
0.9 (0.3-1.2) |
1.1 (0.2-2.1) |
0.8 (0.2-4.5) |
1.5 (0.6-6.2) |
1.6 (0.2-3.9) |
1.1 (0.5-2.1) |
(Note) Reference Value
Except COVID-19 related products
- Reference
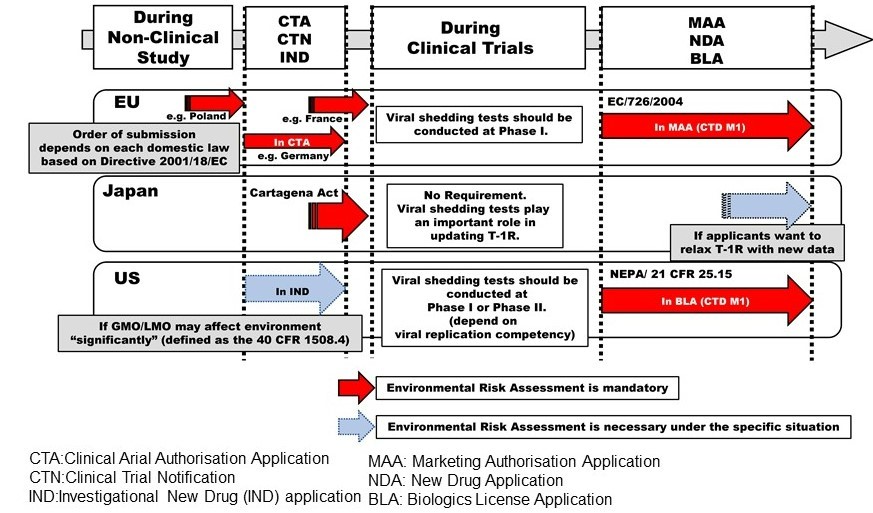
Japanese Pharmaceutical Regulations of Engineered Viral Vectors for Medical Use Compared with those in the United States and the European Union. [external link]
Clin Pharmacol Ther. 2022. doi:10.1002/cpt.2788. PMID: 36404404
Related Notifications and Considerations for GMOs
- Supplementary Explanation on Points to Consider for Preparing Biological Diversity Risk Assessment Report in Application for Approval of Type-1 Use Regulations[458KB]
- Questions and answers (Q&A) on administrative procedures for application for approval, etc. under the Act on the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms[472KB]
- Revision of “Principle of Residual Replication-Incompetent Genetically Modified Viruses Used in the Production of Genetically Engineered Cells”[252KB]
- Evaluation for shedding associated with gene therapy products using viruses/vectors[245KB]
Links
- J-BCH(Japan Biosafety Clearing House) [external link]
Information on “Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms “(Cartagena Protocol domestic law) , related domestic regulations, list of the approved LMO under the law.
- Cartagena Act and related regulations [in J-BCH website]
- Approved Type-1 Use Regulations for Drugs (in Japanese) [in J-BCH website]
- Regenerative Medical Products