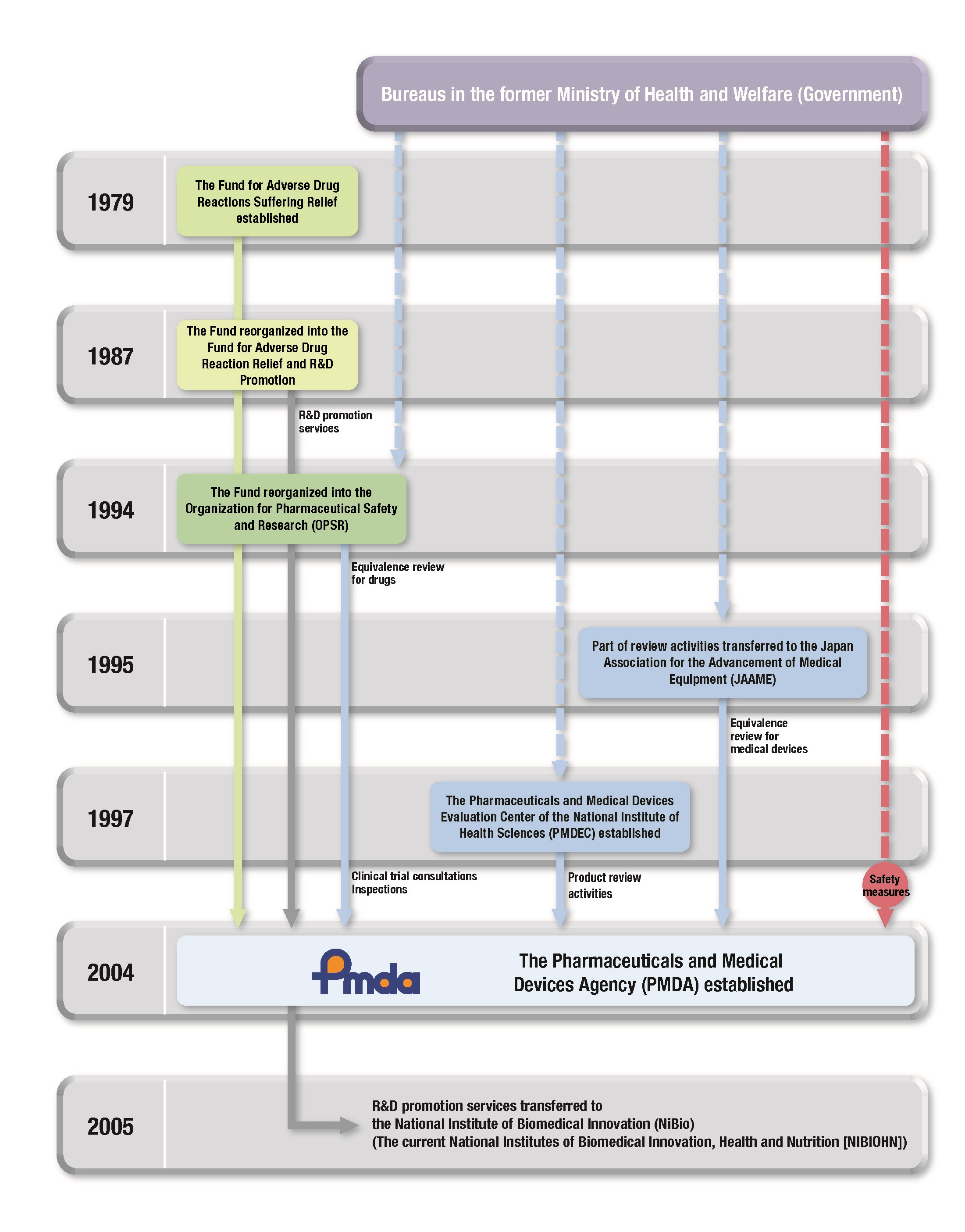
Following the Reorganization and Rationalization Plan for Special Public Corporations that was approved in a Cabinet meeting in 2001, the Pharmaceuticals and Medical Devices Agency (PMDA) was established and came into service on April 1, 2004, under the Law for the Pharmaceuticals and Medical Devices Agency, as a consolidation of the services of the Pharmaceuticals and Medical Devices Evaluation Center of the National Institute of Health Sciences (PMDEC), the Organization for Pharmaceutical Safety and Research (OPSR/KIKO), and part of the Japan Association for the Advancement of Medical Equipment (JAAME).
Back
Back
-
Regulations & Services of PMDAInformation about Approved Products in JapanSafety InformationAdvanced Efforts
-
Information for approved products in JapanSafety information
-
Development of Drugs, Medical devices, Regenerative medicines and in Vitro diagnosticsInformation for approved products in JapanRegulatory science
-
Regulations and services of PMDAInformation for approved products in JapanProcedures in JapanAdvanced efforts
Back
-
Regulations and Services of PMDAInformation for apporoved products in JapanSafety informationProcedures in Japan
-
Regulations and Services of PMDAInformation for apporoved products in JapanSafety informationProcedures in Japan
-
Reviews and Related ServicesRegulatory ProceduresPost-marketing Safety Measures
-
Reviews and Related ServicesPost-marketing Safety Measures
- Home
- About PMDA
- Outline of PMDA
- History
About PMDA