Who We Are
PMDA (Pharmaceuticals and Medical Devices Agency) is Japanese regulatory agency, working together with Ministry of Health, Labour and Welfare.
Our obligation is to protect the public health by assuring safety, efficacy and quality of pharmaceuticals and medical devices.
We conduct scientific reviews of marketing authorization application of pharmaceuticals and medical devices, monitoring of their post-marketing safety. We are also responsible for providing relief compensation for sufferers from adverse drug reaction and infections by pharmaceuticals or biological products.
Name
Pharmaceuticals and Medical Devices Agency (PMDA)
Established
April 1, 2004
Legal classification
Incorporated administrative agency with non-civil service status
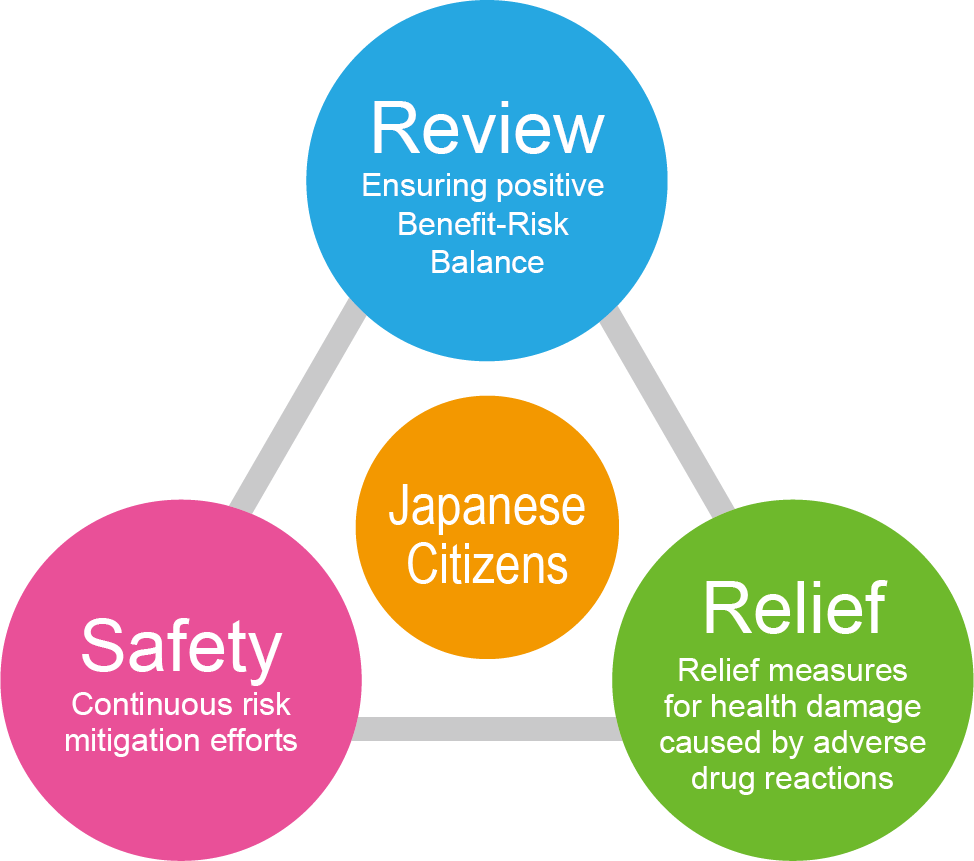
Safety Triangle
Comprehensive Risk Management through the Three Functions
Profile of Services (PMDA's latest brochure)
- Relief Services for Adverse Health Effects
- Relief service for adverse drug reactions
- Relief service for infections acquired through biological products
- Health allowances etc., for SMON patients
- Health allowances for HIV-positive and AIDS patients
- Financial Assistance under the Act on Special Measures concerning the Payment of Benefits to Relieve Patients with Hepatitis C Infection Caused by Specified Fibrinogen Products and Specified Blood Coagulation Factor IX Products
- Reviews
- Consultations on clinical trials and other issues
- Regulatory review of drugs, medical devices and regenerative medical products
- Re-examinations/re-evaluations
- GLP/GCP/GPSP compliance assessments for regulatory submission documentation
- GMP/QMS/GCTP inspections of manufacturing processes and facilities
- Inspection of registered certification bodies
- Development of standards e.g., Japanese Pharmacopoeia
- Post-marketing Safety Measures
- Acceptance of submitted information on precautions
- Collection and organization of safety information from marketing authorization holders (MAHs) or medical institutions
- Scientific research and analysis of collected information
- Consultation services on safety measures for MAHs
- Consultation services for consumers
- Provision of safety information on drugs, medical devices, and regenerative medical products

