PMDA established PMDA Asia Office as its first oversea base
Objectives of establishment of PMDA Asia Office
PMDA Asia Office has been established in Bangkok, the Kingdom of Thailand on 1st, July 2024 as the PMDA’s first oversea base.
PMDA has been providing regulatory trainings for regulatory officials in the Asian region through Asia pharmaceuticals and medical devices training center (PMDA-ATC) and contributed to improve access to the medical products and to strengthen regulatory capacities and regulatory harmonization in Asia. PMDA Asia Office will also contribute further for encouraging harmonisation of pharmaceuticals and medical devices regulation and to assist streamlining regulation for facilitating clinical development in the Asian region towards improved access to innovative pharmaceuticals and medical devices etc. in the Asia region, and hence actively involved in improved health and safety in the Asian countries including Japan.
Ref. Establishment of PMDA Asia Office - as First Overseas Base - (News Release) [266.84KB]
Services of PMDA Asia Office
PMDA Asia Office aims to improve public health in the Asian region by providing the services including development of regulatory cooperation platforms with Asian regulatory authorities, providing direct information exchange on pharmaceutical regulation and various consultations, other related services with companies or organizations expanding in the Asian regions, and local companies or organizations.
Message from the Head of the PMDA Asia Office

I’m Jun Kitahara, Head of PMDA Asia Office. I’ve been involved in international affairs in PMDA for more than 10 years which involves numerous international regulatory harmonization activities such as ICH (Note 1), APEC RHSC (Note 2) and IMDRF (Note 3), liaison between regulatory authority of Switzerland and Japan, management board of WHO collaborating center assigned by WHO. I have a great deal of experience working with relevant international organizations. One of the important aspects of PMDA Asia Office is collaboration with Asian regulatory authorities, and I will utilize my rich experience to improve health and safety of Asian countries including Japan.
(Note 1) International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
(Note 2) Asia-Pacific Economic Cooperation Regulatory Harmonization Steering Committee
(Note 3) International Medical Device Regulators Forum
Head, Pharmaceuticals and Medical Devices Agency (PMDA) Asia Office
Jun Kitahara, Ph.D.
July 2024
Access

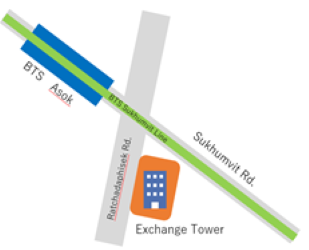
388 Exchange Tower, Unit 2202-2 FL 22, Sukhumvit Road, Khlong Toey Subdistrict, Khlong Toey District, Bangkok 10110, Thailand
International Symposium for Asia Regulatory Coordination
To commemorate the establishment of Asia Office, “International Symposium for Asia Regulatory Coordination” was held. A total of about 120 participants joined the symposium, including H.E. Prof. TAKEMI Keizo, the Minister of Health, Labour and Welfare (at that time), Dr. FUJIWARA Yasuhiro, the Chief executive of PMDA, top level representatives from Asian regulatory agencies, and representatives of industry in Asian region.

For the details of the symposium, please refer to page of International Symposium for Asia Regulatory Coordination.
