Information regarding newly introduced electronic package inserts
Following the amendment of the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (Pharmaceuticals and Medical Devices Act), paper-based package inserts that used to be enclosed with products are abolished in principle from August 2021 Note). Basically, package inserts should be browsed electronically. This page introduces how the electronic package inserts are browsed, and their detailed systems and specifications. The information on this page is current as of 2021 and will be updated in the future.
Note) For products that consumers purchase directly, such as OTC drugs, the paper package inserts will continue to be enclosed.
In particular, for medical devices and in vitro diagnostics, posting the package inserts on the website, which had not been required, is now mandatory. Please refer to the notice following the link shown here for more information [693 KB](only in Japanese). For more information on items subject to electronic package inserts, please refer to the following notification issued by the Ministry of Health, Labour and Welfare (MHLW) "Provision of Information on Precautions, etc. for Drugs, etc.” (PSEHB/PSD Notification No. 0219-1, February 19, 2021), which is listed in the table below.
The marketing authorization holders must register the information on the Safety Information Posting System located on the PMDA's website for the marketing authorization holders. For information on how to register medical devices in the system and post the electronic package inserts, please refer to the information following the link shown here [1,624 KB] (only in Japanese).
Note: The contents of the electronic package inserts and the whole system for browsing such information including the application (app) are available only in Japanese.
How to browse the electronic package inserts (only in Japanese)
Following the amendment of the Pharmaceuticals and Medical Devices Act (Effective on August 1, 2021), an app is available on smartphones, etc. to read the code (barcode or two-dimensional code) on the outer box of a product for direct access to package inserts, etc. This makes it possible to always use the latest information to implement safety measures. From the barcode attached to the box, it is possible to view not only the electronic package inserts, but also related documents such as review reports. Please refer to the following figures.
Note: The contents of the electronic package inserts and the whole system for browsing such information including the app are available only in Japanese.
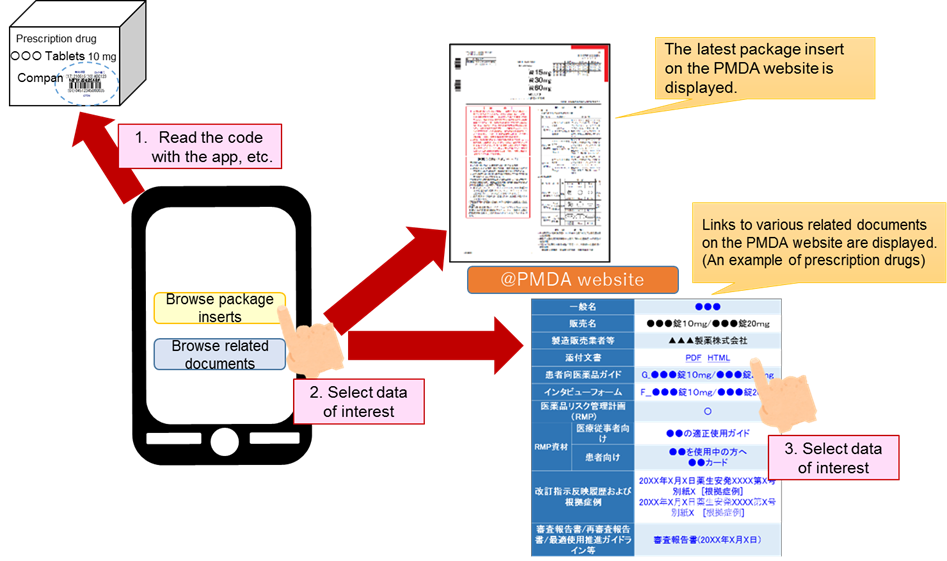
Related documents to be displayed
| Item | Related documents to be displayed |
|---|---|
| Prescription drug products (for healthcare professionals) |
Electronic package inserts, drug guide for patients/guide for vaccinations, drug interview forms, Risk Management Plan (RMP), RMP materials, revision history of PRECAUTIONS and case summary that served as the basis for the revisions, review report/re-examination report/Optimal Clinical Use Guidelines, etc. |
| Prescription drug products (for general consumers) |
Drug guide for patients/guide for vaccinations, drug information sheet, RMP materials (for patients), manuals for management of individual serious adverse drug reactions (ADRs) |
| Medical devices | Electronic package inserts, revision history of PRECAUTIONS, review report/re-examination report, Yellow Letters (Dear Healthcare Professional Letters) |
| In vitro diagnostics | Electronic package inserts, Yellow Letters (Dear Healthcare Professional Letters), Blue Letters, Alert for Proper Use of Drugs |
| Regenerative medical products | Electronic package inserts, review report/summaries of product applications/Optimal Clinical Use Guidelines, etc., Yellow Letters (Dear Healthcare Professional Letters), Blue Letters |
Applications to read the code on the containers of drugs, etc.
PI (Package Insert)-navi (Information in this section is available only in Japanese.)
<Developer>
The Distribution System Research Institute (GS1 Japan), the Federation of Pharmaceutical Manufacturers'
Associations of JAPAN (FPMAJ), the Japan Federation of Medical Devices Associations (JFMDA)
<Download>
The app can be downloaded from the following official stores.
App store
Google play
<Website>
<Related Materials>
Leaflet (brief version) [1,277 KB]
Leaflet (detailed version) [2.1 MB]
Videos
<References>
Administrative notice “Regarding Applications for Smartphones, etc. That Allow Users to Browse the PMDA's Website , Which Contains Information on Precautions, etc., by Reading the Codes on the Containers of Drugs, etc." [1,596 KB]dated May 10, 2021 (only in Japanese)
Technical information on the link between the code on the outer box and the corresponding webpage
Please refer to the following information on how to access the electronic package inserts from the code on the outer box.
Technical details [1,082 KB]
Notifications, etc. related to safety measures such as revisions of package inserts
| Classifications | Date | Title |
|---|---|---|
| Regenerative medical products | June 7, 2024 PSB 0607 No.1 |
Instructions for Electronic Package Inserts of Regenerative Medical Products English-translated notification [218 KB] Original reference in Japanese [192 KB] |
| Regenerative medical products | June 7, 2024 PSB/PSD 0607 No.2 |
Instructions for Electronic Package Inserts of Regenerative Medical Products (Detailed Rules) English-translated notification [175 KB] Original notification in Japanese [218 KB] |
| Drugs | February 17, 2023 PSEHB 0217 No. 1 |
Amendment of the Instructions for Electronic Package Inserts Regarding Results of Studies Using Medical Information English-translated notification [107 KB] Original notification in Japanese [109 KB] |
| Drugs | February 17, 2023 PSEHB/PSD 0217 No.1 |
Points to Consider for Describing the Results of Studies Using Medical Information Database in Electronic Package Inserts English-translated notification [97.1 KB] Original notification in Japanese [104 KB] |
| Regenerative medical products | May 22, 2023 PSEHB 0522 No.1 |
Partial Revision of the "Instructions for Electronic Package Inserts of Regenerative Medical Products" English-translated notification [23.2 KB] Original notification in Japanese [60.8 KB] Reference: Full text after revision (May 22, 2023 Final revision) Instructions for Electronic Package Inserts of Regenerative Medical Products (PSEHB Notification 0611 No.13 June 11, 2021) English-translated reference [55.0 KB] Original reference in Japanese [154 KB] |
| Regenerative medical products | May 22, 2023 PSEHB/PSD 0522 No.1 |
Partial Revision of the "Instructions for Package Inserts of Regenerative Medical Products (Detailed Rules) English-translated notification [27.1 KB] Original notification in Japanese [79.2 KB] Reference: Full text after revision (May 22, 2023 Final revision) Instructions for Package Inserts of Regenerative Medical Products (Detailed Rules) (PFSB/SD Notification 1002 No.13 October 2, 2014) English-translated reference [75.7 KB] Original reference in Japanese [170 KB] |
| Drugs | September 27, 2021 Administrative Notice |
Notice concerning "Standard Workflow for Consideration of Safety Measures such as Revision of Electronic Drug Product Package Inserts" Reference: Standard Workflow for Consideration of Safety Measures |
| Drugs/medical devices/ regenerative medical products/ in vitro diagnostics |
February 19, 2021 Administrative Notice June 11, 2021 (1st Amendment) July 14, 2021 (2nd Amendment) |
Questions and Answers (Qs and As) regarding “Provision of Information on Precautions for Drugs, etc." English-translated administrative notice [19.2 KB] Original administrative notice in Japanese [543 KB] Attachment English-translated attachment [114 KB] Original attachment in Japanese-1 [26.0 KB] Original attachment in Japanese-2 [279 KB] The original Japanese version was established on February 19 and revised on June 11 and July 14, 2021. The English version includes these corrections. |
| Medical devices | May 14, 2021 Administrative Notice |
Points to Consider for Consultations Associated with Revision of Descriptions in Package Inserts, etc. of Medical Devices English-translated administrative notice [58.7 KB] Original administrative notice in Japanese [187 KB] |
| Drugs/medical devices/ regenerative medical products/ in vitro diagnostics |
February 19, 2021 PSEHB/PSD 0219 No.1 |
Provision of Information on Precautions, etc. for Drugs, etc. English-translated notification [129 KB] Original notification in Japanese [139 KB] |
