It all started with the initiative of Professor Mitchell Krucoff of Duke University, who is the incumbent co-chairperson of HBD, when the volunteers from industry, academia and government of both US and Japan got together and discussed on the situation of the clinical trials in Japan which had not been recognized in US at that time. The first face to face meeting took place at the Hotel Chinzanso Tokyo in December 2003, therefore, this year is the commemorable 10th anniversary.
Anyway, it was a great pleasure to have Japan-US HBD East 2013 Think Tank Meeting, however, it was worth mentioning that we had been all set for holding the Japan- US HBD East 2011 Think Tank Meeting in March 2011, which we had to cancel because of the big damage to Japan caused by the Great East Japan Earthquake and subsequent catastrophic Tsunami and sporadic after-quakes. We greatly appreciate warm sympathy and various support extended to us since then from so many people around the world. Japan has been making steady recovery since then, thanks to all those support.
Although the Think Tank Meeting that year had to be canceled, the activities have been resumed by the four working groups as the central body of HBD. The members have kept communicating closely and made remarkable progress. Various achievements by four working groups were reported to the audience at this Think Tank Meeting.
The program, attached hereto, covered not only progress reports from each working group above but also those topics like Scientific Session, future vision for global cooperation and total product life cycle management, which were presented by well-balanced representatives of industries, academia and regulators.
About two hundred people including HBD members attended the meeting, showing great interest in our activities. While it was unfortunate that some of the visitors from US including a speaker had to cancel their trip to Japan due to the tragic accident at San Francisco International Airport on the previous day, it was a great relief that Dr. Kentaro Hayashida of Keio University took his time to make a presentation for the speaker who could not make it to Japan. We would like to express our deepest gratitude to Dr. Hayashida.
From the presentation of Ms. Kimberly Trautman, who attended in person as the sole representative from FDA, and from the video messages contributed from Dr. Jeffrey Shuren, Director of CDRH, and Dr. Ken Cavanaugh, also from CDRH, we understand it is FDA’s intention to continue vigorous discussion on the cooperation between US and Japan based on HBD, and we agreed to continue to promote HBD activities focusing on new WG1 and WG2.
The Steering Committee had named the subtitle of this Think Tank Meeting as “Past Decade, Current and Tomorrow”, in hope of discussing future direction of this international collaboration along with the achievements in the past. Looking back the past ten years, it may seem somewhat long and somewhat short. The activities have been supported by so many people, some worked all through those ten years, some worked on temporary basis and some early contributors who can no longer participate due to the change of their jobs. Therefore, the presentations for today and tomorrow are the fruits of the efforts rendered not only by the current members but also by other people involved in this activity in the past ten years. As the co-chairperson of HBD, I would like to take this opportunity to express our sincere gratitude for those who could not attend this meeting.
Meanwhile, the Japanese Government has announced the strategies for enhancing health and medical care in June, and in that statement the following key messages were delivered. In order to contribute to the development of world-class, innovative pharmaceuticals, medical devices and cellular and tissue-based products, utilization of research results will be promoted and the guidelines for review process for innovative pharmaceuticals and medical devices, which shall be aligned with international standards, will be established. Also, international harmonization of review process will be promoted, while the relevant authorities of Japan, US, EU and so on will continue to exchange their opinions in order to discuss on (possible collaboration for) review, consultation, GCP or on-site inspection. In particular, for medical devices, international concurrent development will be pursued, taking advantage of such activities like HBD between the authorities of US and Japan. These statements clearly indicate that the Japanese government recognizes HBD as a nationally important activity.
The environment surrounding medical devices is rapidly changing, especially, in terms of regulation, US and EU tend to tighten it up while emerging countries are trying to establish of their own. Therefore, the framework of the collaboration built so far by industries, academia and regulators of US and Japan may need to change in due course. In this context, I ask all of you to vigorously discuss during this meeting, so that we can establish the future direction based upon your precious input.
Finally, we would like to express cordial gratitude to all the attendees who have made this Think Tank Meeting a great success. We sincerely hope that the fruits of this meeting will contribute to the public health of not only US and Japan but also of the world.
HBD co-Chairs
Mitchell Krucoff
Atsushi Tamura
Scenes from Think Tank Meeting
 |
 |
| Opening Remarks | Dr. Kondo, Chief Executive of PMDA |
 |
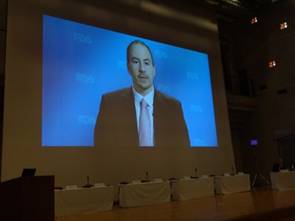 |
| Dr. Shuren, Director of CDRH | Dr. Cavanaugh, Vascular Surgery Devices Branch of CDRH |
 |
 |
| Panel Discussion (From left, Dr. Hayashida of Keio U., who ‘pinch-hit’ for the absent speaker as the result of the crash at SFO, Dr. Krucoff of Duke U., Dr. Sawa of Osaka U. and Dr. Shirato of PMDA) |
HBD members assembled |
