厚生労働省/PMDAは、日本国民の健康と安全の向上、ひいては世界の保健衛生の向上のために、世界各国の規制当局等と協働しています。
世界の規制当局等と厚生労働省/PMDAとの関わり
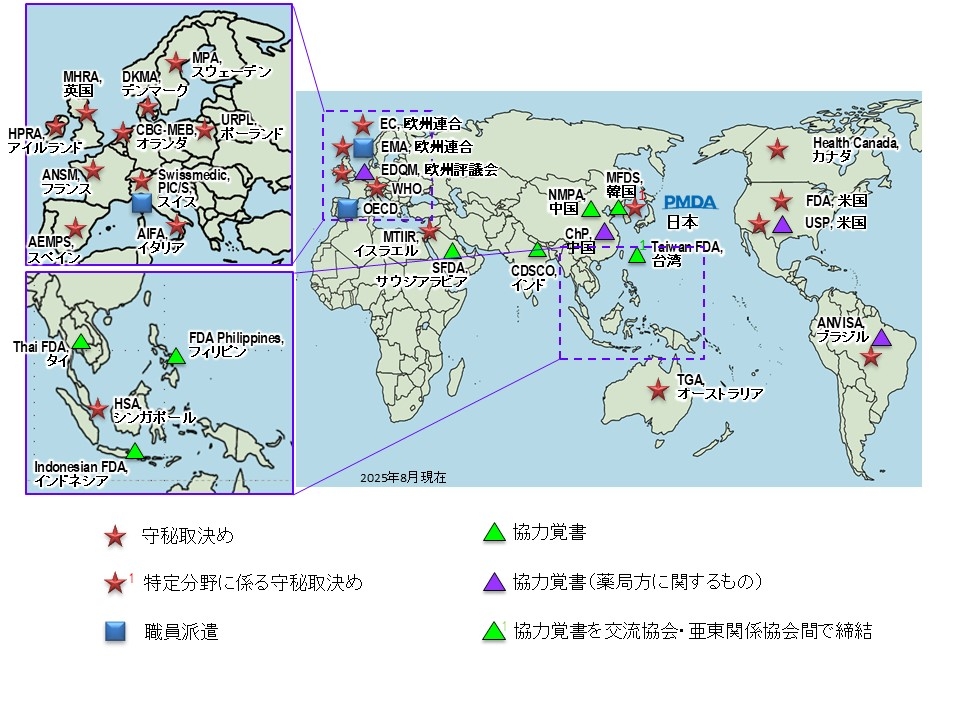
守秘に関する交換文書
協力覚え書 (Memorandum of Understanding/Memorandum of Cooperation)
略語
EU: European Union
UK: United Kingdom of Great Britain and Northern Ireland
USA: United States of America
AEMPS: Agencia Española de Medicamentos y Productos Sanitarios (The Spanish Agency of Medicines and Medical Devices)
AIFA: Agenzia Italiana del Farmco (The Italian Medicines Agency)
ANSM: L'Agence Nationale de Sécurité du Médicament et des produits de santé (The French National Agency for Medicines and Health Products Safety)
ANVISA: Agência Nacional de Vigilância Sanitária (The Brazilian Health Regulatory Agency)
CDSCO: Central Drugs Standard Control Organization
CFDA: China Food and Drug Administration
ChP: Chinese Pharmacopoeia Commission
DKMA: Danish Medicines Agency
EC: European Commission
EDQM: European Directorate for the Quality of Medicines & HealthCare
EMA (Former EMEA): European Medicines Agency
FDA: Food and Drug Administration
FDA Philippines: Food and Drug Administration Philippines
HPRA: Health Products Regulatory Authority
HSA: Health Sciences Authority
Indonesian FDA: Indonesian Food and Drug Authority
IMB: The Irish Medicines Board
MEB: Medicines Evaluation Board
MFDS: Ministry of Food and Drug Safety
MHRA: Medicines and Healthcare products Regulatory Agency
MPA: Medical Products Agency
MTIIR: Medical Technology, Health Information, Innovation and Research
NMPA: National Medical Products Administration
NPRA: National Pharmaceutical Regulatory Agency
SFDA1: State Food and Drug Administration
SFDA2: Saudi Food and Drug Authority
TGA: Therapeutic Goods Administration
URPLWMiPB: Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych (Office for Registration of Medicinal Products, Medical Devices and Biocidal Products)
USP: United States Pharmacopeial convention
WHO: World Health Organization
MHLW: Ministry of Health, Labour and Welfare
PMDA: Pharmaceuticals and Medical Devices Agency
